��Ŀ����
�������� HClO Ka=3.0��10-8��HNO2 Ka=4.6��10-4��HCN Ka=4.93��10-10
̽��1����С�ձ��м���20mL0.1mol/LFeCl3����pH�Ʋ�����Һ��pH������һֻС�ձ��м���5mL0.1mol/LFeCl3��Һ��������ˮϡ����50mL����pH�Ʋ�������Һ��pH����̽���õ��Ľ�����д������Ŀո��У�
̽��2����A��B��֧�Թ��зֱ��������0.1mol/LFe2��SO4��3��Һ����A�Թ��ھƾ��ƻ����ϼ��ȵ����ڣ�B�Թܱ������£��Ʋ�A��B���Թ�����ʢ��ɢϵ����ɫ�IJ�𣮽�̽���õ��Ľ�����д������Ŀո��У�
̽��3��ȡA��B��ֻС�ձ�����A���м���20mL����ˮ����B���м���20mL10mol/L��HCl���ٷֱ�����ֻС�ձ���Ͷ��2.0gFeCl3���壬�ò���������ʹ�����ܽ⣮A�ձ����ֻ��ǣ�B�ձ�Ϊ������Һ����̽���õ��Ľ��ۼ��ý��۵�Ӧ��֮һ��д������Ŀո��У�
̽��4��ȡ��֧�Թܣ��ֱ����10mL0.1mol/LNaCN��NaClO��NaNO2��Һ����pH����������Һ��pH���Ʋ���������ҺpH����Դ�С����̽���õ��Ľ�����д������Ŀո��У� �ش���������
��1�������ĸ�̽����̽��������
��2���뽫��С��ÿ��̽�����õ��Ľ��ۣ��еĻ�Ҫ��д���۵�Ӧ�ã����ڶ�Ӧ�Ŀո��У�
̽��1
̽��2
̽��3
Ӧ�þ���
̽��4
��2��̽��1���Ȼ�����Һϡ�ͣ�Ũ�ȼ�С��ҺpH������Һ���ֻ��ǣ�
̽��2��A�Թ��г��ֺ��ɫ���� B�ػ�ɫ��Һ�ޱ仯��
̽��3��������ˮ������ԣ������������������ӵ�ˮ�⣻
Ӧ�þ��������������ӵ�ˮ��������ԣ�ˮ����������ȷ�Ӧ��
̽��4����������ĵ���ƽ�ⳣ����С������ƽ�ⳣ��ԽС����Ӧ�������Խ�������ɵ���ˮ��̶�Խ��
�ʴ�Ϊ��̽������ˮ��ƽ���Ӱ�����أ�
��2��̽��1���Ȼ�����Һϡ�ͣ�Ũ�ȼ�С��ҺPH����˵���ε�ˮ���Ũ���йأ��ε�Ũ��Խ����Һ������Խǿ��
�ʴ�Ϊ���ε�Ũ��Խ����Һ����Խǿ��
̽��2��A�Թ��г��ֺ��ɫ���� B�ػ�ɫ��Һ�ޱ仯������ˮ�������ȹ��̣��¶�Խ�ߣ��Ȼ���ˮ��̶�Խ������Խǿ��
�ʴ�Ϊ���¶�Խ�ߣ��Ȼ���ˮ��̶�Խ������Խǿ��
̽��3��������ˮ������ԣ������������������ӵ�ˮ�⣬������Һ�п��������ε�ˮ�⣬
�ʴ�Ϊ��������Һ�п��������ε�ˮ�⣻
Ӧ�þ���������FeCl3��Һ�����Խ��Ȼ����������ܽ���Ũ������Һ�У��ټ�����ˮϡ�͵�����Ũ�ȣ�
�ʴ�Ϊ������FeCl3��Һ�����Խ��Ȼ����������ܽ���Ũ������Һ�У��ټ�����ˮϡ�͵�����Ũ�ȣ�
̽��4����������ĵ���ƽ�ⳣ����С������HClO Ka=3.0��10-8��HNO2 Ka=4.6��10-4��HCN Ka=4.93��10-10��ƽ�ⳣ��ԽС����Ӧ�������Խ�������ɵ���ˮ��̶�Խ�ʴ�Ϊ����ˮ�����ɵ���Խ�����ε�ˮ��̶�Խ��

ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣺
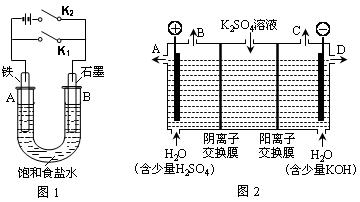
��.��ͼ1��ʵ��װ�ý���ʵ�顣
��1������ʼʱ����K2���պϿ���K1�����װ��Ϊ ����װ�����ƣ���
��2������ʼʱ����K1���պϿ���K2����
��У2010�������ҵ����ĩ������ѧ�Ծ� ��7ҳ ����12ҳ��
��U�����ܷ�Ӧ�����ӷ���ʽΪ 
�ڶ�������ʵ�飬����˵����ȷ���� ������ţ���
| A����Һ��Na+��B���ƶ� |
| B����A�����ݳ���������ʹʪ���KI������ֽ���� |
| C����Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ�� |
| D������״���´�A���ݳ�2.24L���壬�����·��ͨ���ĵ�����Ŀ�Դ���0.2NA |
��3����С��ͬѧ��Ϊ�����ģ�ҵ�����ӽ���Ĥ�����ռ�ķ�������ô������������ͼ2װ�õ���������Һ����ȡ������������������������أ������ڵ������ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ������
�ٸõ��۵�������ӦʽΪ ����ʱͨ�������ӽ���Ĥ�������� ������ڡ�����С�ڡ����ڡ���ͨ�������ӽ���Ĥ����������
���Ƶõ�����������Һ�ӳ��� ���A������B������C������D����������
��ͨ�翪ʼ������������Һ��pH�����������ԭ�� ��
ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣺
 |
��.��ͼ1��ʵ��װ�ý���ʵ�顣
��1������ʼʱ����K2���պϿ���K1�����װ��Ϊ ����װ�����ƣ���
��2������ʼʱ����K1���պϿ���K2����
��У2010�������ҵ����ĩ������ѧ�Ծ� ��7ҳ ����12ҳ��
��U�����ܷ�Ӧ�����ӷ���ʽΪ
�ڶ�������ʵ�飬����˵����ȷ���� ������ţ���
A. ��Һ��Na+��B���ƶ�
B. ��A�����ݳ���������ʹʪ���KI������ֽ����
C. ��Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ��
D. ����״���´�A���ݳ�2.24L���壬�����·��ͨ���ĵ�����Ŀ�Դ���0.2NA
��.��ͼ2��ʵ��װ�ý���ʵ�顣
��3����С��ͬѧ��Ϊ�����ģ�ҵ�����ӽ���Ĥ�����ռ�ķ�������ô������������ͼ2װ�õ���������Һ����ȡ������������������������أ������ڵ������ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ������
�ٸõ��۵�������ӦʽΪ ����ʱͨ�������ӽ���Ĥ�������� ������ڡ�����С�ڡ����ڡ���ͨ�������ӽ���Ĥ����������
���Ƶõ�����������Һ�ӳ��� ���A������B������C������D����������
��ͨ�翪ʼ������������Һ��pH�����������ԭ�� ��
ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣺
 |
��.��ͼ1��ʵ��װ�ý���ʵ�顣
��1������ʼʱ����K2���պϿ���K1�����װ��Ϊ ����װ�����ƣ���
��2������ʼʱ����K1���պϿ���K2����
��У2010�������ҵ����ĩ������ѧ�Ծ� ��7ҳ ����12ҳ��
��U�����ܷ�Ӧ�����ӷ���ʽΪ
�ڶ�������ʵ�飬����˵����ȷ���� ������ţ���
A. ��Һ��Na+��B���ƶ�
B. ��A�����ݳ���������ʹʪ���KI������ֽ����
C. ��Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ��
D. ����״���´�A���ݳ�2.24L���壬�����·��ͨ���ĵ�����Ŀ�Դ���0.2NA
��.��ͼ2��ʵ��װ�ý���ʵ�顣
��3����С��ͬѧ��Ϊ�����ģ�ҵ�����ӽ���Ĥ�����ռ�ķ�������ô������������ͼ2װ�õ���������Һ����ȡ������������������������أ������ڵ������ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ������
�ٸõ��۵�������ӦʽΪ ����ʱͨ�������ӽ���Ĥ�������� ������ڡ�����С�ڡ����ڡ���ͨ�������ӽ���Ĥ����������
���Ƶõ�����������Һ�ӳ��� ���A������B������C������D����������
��ͨ�翪ʼ������������Һ��pH�����������ԭ�� ��