��Ŀ����
�±������������г��������ʣ������г������ǵģ���Ҫ���ɷ֣�
��1������Ա��Т١��ߵ���Ҫ�ɷֽ��з��ࣨ���ţ���
�������
��2��д�������ڵ�ˮ��Һ��߷�Ӧ�Ļ�ѧ����ʽ
��3��ijͬѧ�âݺ�Ũ���Ṳ�����Ʊ��ޣ���ѧ����ʽΪ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O
��������ת�Ƶķ������Ŀ��������ת��0.1molʱ�����Ļ�ԭ��������Ϊ
| ��� | �� | �� | �� | �� | �� | �� | �� |
| ���� | �ƾ� | �� | ��� | ʳ�� | ͭ���� | �������� | �մ� |
| ��Ҫ �ɷ� |
CH3CH2OH | CH3COOH | NaOH | NaCl | Cu | SO2 | Na2CO3 |
�������
�ܢ�
�ܢ�
�����ڵ���ʵ����ڢۢܢ�
�ڢۢܢ�
�����ڷǵ���ʵ����٢�
�٢�
����2��д�������ڵ�ˮ��Һ��߷�Ӧ�Ļ�ѧ����ʽ
2CH3COOH+Na2CO3=2CH3COONa+CO2��+H2O
2CH3COOH+Na2CO3=2CH3COONa+CO2��+H2O
������������߷�Ӧ�����ӷ���ʽH++CO32-=HCO3-
H++CO32-=HCO3-
����3��ijͬѧ�âݺ�Ũ���Ṳ�����Ʊ��ޣ���ѧ����ʽΪ��Cu+2H2SO4��Ũ��
| ||
��������ת�Ƶķ������Ŀ��������ת��0.1molʱ�����Ļ�ԭ��������Ϊ
3.2
3.2
g����������1���ܵ�������������ӣ���笠����ӣ���������ӵĻ����������Σ���ˮ��Һ������״̬���ܵ���Ļ�����е���ʣ���ˮ��Һ������״̬�¾����ܵ���Ļ�����зǵ���ʣ�
��2���������ʵ�������д��ѧ����ʽ�����ӷ���ʽ��
��3����Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��Ӧ�У�Cu���ϼ����ߣ���0�����ߵ�+2�ۣ�SԪ�ػ��ϼ۽��ͣ���+6�۽��͵�+4�ۣ����ݷ���ʽÿ��Ӧ1molͭת��2mol���ӣ�
��2���������ʵ�������д��ѧ����ʽ�����ӷ���ʽ��
��3����Cu+2H2SO4��Ũ��
| ||
����⣺��1���ܢ����ܵ�������������Ӻ�������ӵĻ�����������࣬�ڢۢܢ�����ˮ��Һ״̬�¾��ܵ���Ļ�������ڵ���ʣ��٢�����ˮ��Һ������״̬�¾����ܵ���Ļ�������ڷǵ���ʣ��ʴ�Ϊ���ܢߣ��ڢۢܢߣ��٢ޣ�
��2��CH3COOH��̼���Ʒ�Ӧ���ɴ����ƺͶ�����̼������ʽΪ2CH3COOH+Na2CO3=2CH3COONa+CO2��+H2O��̼���ƺ��������ᷴӦ�����Ȼ��ơ�̼�����ƣ���Ӧ�����ӷ���ʽΪH++CO32-=HCO3-���ʴ�Ϊ��2CH3COOH+Na2CO3=2CH3COONa+CO2��+H2O��H++CO32-=HCO3-��
��3���⣺ͭʧ������=1��2-0��=2������õ�����=1��6-4��=2���÷�Ӧ��ת�Ƶ�������2������˫���ű���÷�Ӧ����ת�Ƶķ������ĿΪ�� ��Cu���ϼ����ߣ���0�����ߵ�+2�ۣ�SԪ�ػ��ϼ۽��ͣ���+6�۽��͵�+4�ۣ����ݷ���ʽÿ��Ӧ1molͭת��2mol���ӣ�������ת��0.1molʱ����Ӧ��ͭΪ0.05mol������Ϊ3.2g���ʴ�Ϊ��
��Cu���ϼ����ߣ���0�����ߵ�+2�ۣ�SԪ�ػ��ϼ۽��ͣ���+6�۽��͵�+4�ۣ����ݷ���ʽÿ��Ӧ1molͭת��2mol���ӣ�������ת��0.1molʱ����Ӧ��ͭΪ0.05mol������Ϊ3.2g���ʴ�Ϊ��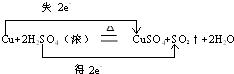 ��3.2��
��3.2��
��2��CH3COOH��̼���Ʒ�Ӧ���ɴ����ƺͶ�����̼������ʽΪ2CH3COOH+Na2CO3=2CH3COONa+CO2��+H2O��̼���ƺ��������ᷴӦ�����Ȼ��ơ�̼�����ƣ���Ӧ�����ӷ���ʽΪH++CO32-=HCO3-���ʴ�Ϊ��2CH3COOH+Na2CO3=2CH3COONa+CO2��+H2O��H++CO32-=HCO3-��
��3���⣺ͭʧ������=1��2-0��=2������õ�����=1��6-4��=2���÷�Ӧ��ת�Ƶ�������2������˫���ű���÷�Ӧ����ת�Ƶķ������ĿΪ��
 ��Cu���ϼ����ߣ���0�����ߵ�+2�ۣ�SԪ�ػ��ϼ۽��ͣ���+6�۽��͵�+4�ۣ����ݷ���ʽÿ��Ӧ1molͭת��2mol���ӣ�������ת��0.1molʱ����Ӧ��ͭΪ0.05mol������Ϊ3.2g���ʴ�Ϊ��
��Cu���ϼ����ߣ���0�����ߵ�+2�ۣ�SԪ�ػ��ϼ۽��ͣ���+6�۽��͵�+4�ۣ����ݷ���ʽÿ��Ӧ1molͭת��2mol���ӣ�������ת��0.1molʱ����Ӧ��ͭΪ0.05mol������Ϊ3.2g���ʴ�Ϊ�� ��3.2��
��3.2�����������⿼���Ϊ�ۺϣ��漰���ʵķ����������ԭ��Ӧ��֪ʶ����Ŀ�ѶȲ�����ȷԪ�ػ��ϼ۱仯�ǽⱾ��ؼ����ѵ��DZ����ת�Ƶķ������Ŀ��ע���ͷ��ָ��

��ϰ��ϵ�д�
 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
�����Ŀ
(11��)�±������������г��������ʣ������г������ǵģ���Ҫ���ɷ֡�
|
��� |
�� |
�� |
�� |
�� |
�� |
�� |
�� |
|
���� |
�ƾ� |
���� |
��� |
ʳ�� |
ͭ���� |
�������� |
�մ� |
|
��Ҫ �ɷ� |
CH3CH2OH |
CH3COOH |
NaOH |
NaCl |
Cu |
SO2 |
Na2CO3 |
��1������Ա��Т١��ߵ���Ҫ�ɷֽ��з��ࣨ���ţ���
�����ε��� �����ڵ���ʵ��� �����ڷǵ���ʵ��� ��
��2��д�������ڵ�ˮ��Һ��߷�Ӧ�Ļ�ѧ����ʽ ��
����������߷�Ӧ�����ӷ���ʽ ��
��ijͬѧ�âݺ�Ũ���Ṳ�����Ʊ��ޣ���ѧ����ʽΪ��
Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
��������ת�Ƶķ������Ŀ��������ת��0.1molʱ�����Ļ�ԭ��������Ϊ g��

