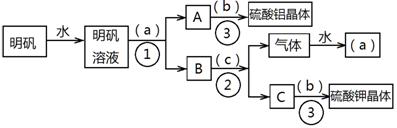
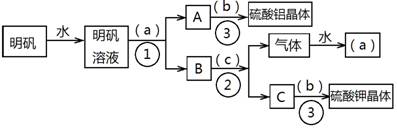
��Ŀ����
��ͼ��������KAl(SO4)2•12H2O����ȡ������������صIJ�����������ͼ��ͼ����ȥ�˳������ϴ�Ӳ�����������ͼ��Բ�����������ʵ����Լ����ƣ��ڷ������������ʵ��ķ��뷽�������ش��й����⡣
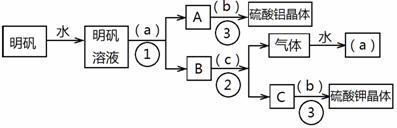
��1���Լ�(a)��________________��(b)��______________��(c)��____________��
��2�����뷽������________________������____________________;������������Ҫ�õ��IJ���������_______________________________��
��3����������������������ģ���������158g������Ħ������474g/mol���������Ƶ����������塲Al2(SO4)3•18H2O��(Ħ������666g/mol) _____g�����ٿ����Ʊ�K2SO4(Ħ������174g/mol)_______g��
��1����a����ˮ;��b������;��c���������ء�
��2���ٹ���;���������ᾧ��;�ձ�����������©�����ƾ��ơ�
��3��66.6g; 69.6g��

��ϰ��ϵ�д�
 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д�
�����Ŀ