��Ŀ����
��8�֣�ij����С����MnO2��Ũ�����Ʊ�Cl2ʱ�����ø����չ�����SO2��NaOH��Һ����β���������մ�����
��1�������SO2�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�� ________________��
��2����ӦCl2+Na2SO3+2 NaOH===2NaCl+Na2SO4+H2O�е���������Ϊ______________��
��3�������MnO2��Ũ�����Ʊ�Cl2�����ӷ���ʽ�� _______ _________��
��4�����14.2g������������������Ӧ��Ȼ�����ɵ�������500mLijŨ�ȵ�NaOH��Һǡ�÷�Ӧ�����NaOH��Ũ��Ϊ ����������Һ������䣩
��1�������SO2�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�� ________________��
��2����ӦCl2+Na2SO3+2 NaOH===2NaCl+Na2SO4+H2O�е���������Ϊ______________��
��3�������MnO2��Ũ�����Ʊ�Cl2�����ӷ���ʽ�� _______ _________��
��4�����14.2g������������������Ӧ��Ȼ�����ɵ�������500mLijŨ�ȵ�NaOH��Һǡ�÷�Ӧ�����NaOH��Ũ��Ϊ ����������Һ������䣩
��1��SO2 + 2 NaOH="== " Na2SO3 + H2O
��2��Na2SO3
��3��MnO2 + 2Cl +4H+ ="== " Cl2 + Mn2+ + 2H2O
+4H+ ="== " Cl2 + Mn2+ + 2H2O
��4��0.8mol/L ��2�֣�
��2��Na2SO3
��3��MnO2 + 2Cl
 +4H+ ="== " Cl2 + Mn2+ + 2H2O
+4H+ ="== " Cl2 + Mn2+ + 2H2O��4��0.8mol/L ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 I2��HNO3��NO
I2��HNO3��NO �����Դ�ǿ������˳���� ��
�����Դ�ǿ������˳���� �� MnCl2��C12����2H2O�У�����0.2mol����ת��ʱ�������������������״������ ��
MnCl2��C12����2H2O�У�����0.2mol����ת��ʱ�������������������״������ ��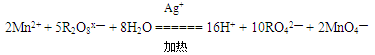
 2Cu+SO2����˵����ȷ���� �� ��
2Cu+SO2����˵����ȷ���� �� �� ��������
��������