��Ŀ����
�����£���һԪ��HA��Һ��NaOH��Һ�������ϣ�ʵ�����������
����˵����ȷ����
A��ʵ��ٷ�ӦǰHA��Һ��c(H+)��c(OH��)+ c(A��)
B��ʵ��ٷ�Ӧ����Һ��c(A��)��c(Na +)
C��ʵ��ڷ�ӦǰHA��ҺŨ��x��0.2 mol��L��1
D��ʵ��ڷ�Ӧ����Һ��c(A��)+ c(HA)= c(Na+)
| ʵ���� | ��ʼŨ��c(HA) | ��ʼŨ��c(NaOH) | ��Ӧ����Һ��pH |
| �� | 0.1 mol��L��1 | 0.1 mol��L��1 | 9 |
| �� | x | 0.2mol��L��1 | 7 |
����˵����ȷ����
A��ʵ��ٷ�ӦǰHA��Һ��c(H+)��c(OH��)+ c(A��)
B��ʵ��ٷ�Ӧ����Һ��c(A��)��c(Na +)
C��ʵ��ڷ�ӦǰHA��ҺŨ��x��0.2 mol��L��1
D��ʵ��ڷ�Ӧ����Һ��c(A��)+ c(HA)= c(Na+)
AC
���������A����Ӧǰ��HA������� ����H+��A����������Һ�е�H+����HA��������ĺ�ˮ��������ģ���ˮ���������H+��OH����ȣ����Է�ӦǰHA��Һ��c(H+)��c(OH��)+ c(A��)����ȷ��B��ʵ��ٷ�Ӧ����ҺΪNaA��Һ������A��ˮ��ʹ��Һ�ʼ��ԣ�����c(A��)<c(Na +)������C�����������Ũ�ȵ�����Ӧ�����ҺΪ���ԣ���ʹ��Һ�����ԣ�������ʵ���Ӧ���ڼ����x��0.2 mol��L��1����ȷ��D��ʵ��ڷ�Ӧ����Һ�����ԣ����ݵ���غ㣬��c(A��)= c(Na+)������ѡAC��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
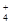 ��I����SCN��
��I����SCN��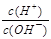 ��1��10��12����Һ�� K����AlO2����CO32����Na���������ӿ��Դ�������
��1��10��12����Һ�� K����AlO2����CO32����Na���������ӿ��Դ�������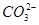 ��
��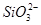 ��
��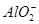 ��Cl��
��Cl��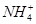 ��Na��
��Na��
 Sn(OH)2
Sn(OH)2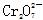 ��CH3CH2OH��
��CH3CH2OH��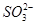
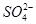
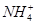 ��
��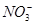 ��Cl��
��Cl��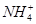 )/c(NH3��H2O)��ֵ����
)/c(NH3��H2O)��ֵ����