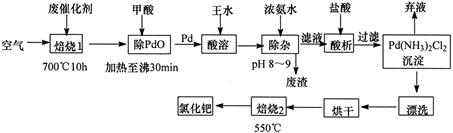
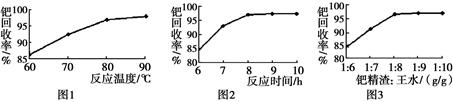
��Ŀ����
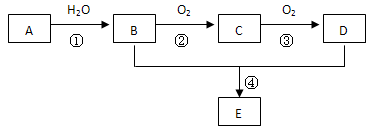
 ���㻯����A��B��Ϊͬ���칹��,B�Ľṹ��ʽ�� CH3COO�� ��COOCH2CH3 ,A���١���������Ӧ��C��D��E��B���١���������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ����ͼ��ʾ:
���㻯����A��B��Ϊͬ���칹��,B�Ľṹ��ʽ�� CH3COO�� ��COOCH2CH3 ,A���١���������Ӧ��C��D��E��B���١���������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ����ͼ��ʾ: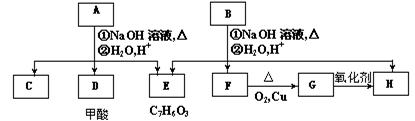
(1)B���Է����ķ�Ӧ������(�����)_______________.
�ټӳɷ�Ӧ ��������Ӧ ����ȥ��Ӧ ��ȡ����Ӧ �ݾۺϷ�Ӧ
B�ĺ˴Ź��������л����_______����.
(2)E�к��й����ŵ�������__________________.
(3)A�����ֿ��ܵĽṹ,���Ӧ�Ľṹ��ʽΪ________________��______________.
(4)B��C��D��F��G�������л�Ϊͬϵ�����_________________.
(5)F��H��Ũ���������¼���ʱ������Ӧ�ķ���ʽΪ_________________________.
��1���٢ܣ�2�֣� 5��2�֣���2���ǻ����Ȼ�����1�֣�
��3��HCOO�� ��COOCH2CH2CH3��2�֣� HCOO��
��COOCH2CH2CH3��2�֣� HCOO�� ��COOCH2(CH3)2��2�֣�
��COOCH2(CH3)2��2�֣�
��4��C F ��2�֣� ��5��CH3COOH + CH3CH2OH CH3COOCH2CH3 + H2O��3�֣�
CH3COOCH2CH3 + H2O��3�֣�
��3��HCOO��
 ��COOCH2CH2CH3��2�֣� HCOO��
��COOCH2CH2CH3��2�֣� HCOO�� ��COOCH2(CH3)2��2�֣�
��COOCH2(CH3)2��2�֣���4��C F ��2�֣� ��5��CH3COOH + CH3CH2OH
 CH3COOCH2CH3 + H2O��3�֣�
CH3COOCH2CH3 + H2O��3�֣���1������B�Ľṹ��ʽ��֪��B�к��б����������������ܷ����ķ�Ӧ�Ǽӳɺ�ȡ������ѡ�٢ܡ����ڲ����Ƕ�λ��ϵ�����Թ�����5�ֲ�ͬ���͵���ԭ�ӡ�
��2������B�Ľṹ��ʽ��֪��Bˮ��IJ���ֱ������ᡢ���ǻ���������Ҵ�������F���Ҵ���G����ȩ��H�����ᣬE�Ƕ��ǻ������ᡣ����E�к��й����ŵ��������ǻ����Ȼ���
��3��A�����ֿ��ܵĽṹ�����Ը���B�Ľṹ��ʽ��֪�����ֽṹ�ֱ���HCOO�� ��COOCH2CH2CH3�� HCOO��
��COOCH2CH2CH3�� HCOO�� ��COOCH2(CH3)2��
��COOCH2(CH3)2��
��4���ṹ���ƣ��������������ɸ�CH2ԭ���ŵ�ͬһ�����ʻ�Ϊͬϵ�����C��F��Ϊͬϵ���ΪC�DZ�����
(5)F��H��Ũ���������¼��ȷ���������Ӧ������ʽΪCH3COOH + CH3CH2OH CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O
��2������B�Ľṹ��ʽ��֪��Bˮ��IJ���ֱ������ᡢ���ǻ���������Ҵ�������F���Ҵ���G����ȩ��H�����ᣬE�Ƕ��ǻ������ᡣ����E�к��й����ŵ��������ǻ����Ȼ���
��3��A�����ֿ��ܵĽṹ�����Ը���B�Ľṹ��ʽ��֪�����ֽṹ�ֱ���HCOO��
 ��COOCH2CH2CH3�� HCOO��
��COOCH2CH2CH3�� HCOO�� ��COOCH2(CH3)2��
��COOCH2(CH3)2����4���ṹ���ƣ��������������ɸ�CH2ԭ���ŵ�ͬһ�����ʻ�Ϊͬϵ�����C��F��Ϊͬϵ���ΪC�DZ�����
(5)F��H��Ũ���������¼��ȷ���������Ӧ������ʽΪCH3COOH + CH3CH2OH
 CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O
��ϰ��ϵ�д�
�����Ŀ

 ��������ͨ����ͬ�ķ�Ӧ�õ��������ʣ�
��������ͨ����ͬ�ķ�Ӧ�õ��������ʣ�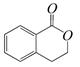 C��
C��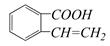
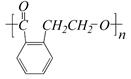 E��
E��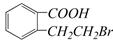
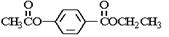 ��A���١���������Ӧ��C��D��E ��B���١���������Ӧ��E��F��H ��������Ӧ���̡��������ʼ����ϵ��ͼ��ʾ��
��A���١���������Ӧ��C��D��E ��B���١���������Ӧ��E��F��H ��������Ӧ���̡��������ʼ����ϵ��ͼ��ʾ��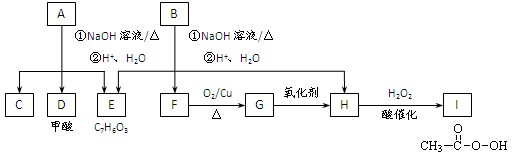
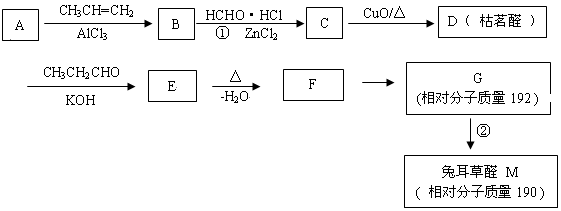
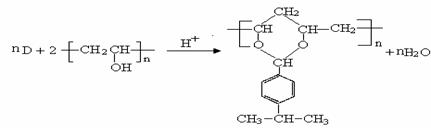
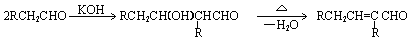
 CH3CHO��
CH3CHO��