��Ŀ����
����Ŀ�����ڹ�����ռ����Ҫ��λ��
(1)��֪��![]()
![]()
![]()
![]()
![]()
![]()
��![]() ��
��![]() ________��
________��
(2)![]() �Ļ��
�Ļ��![]() ���ֽⷴӦ��
���ֽⷴӦ��![]() �Ļ��
�Ļ��![]() ________��
________��
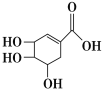
(3)�ҹ���ѧ����![]() Ϊ�������ڲ�ͬ�������Һ��ʵ�ֳ��µ���ϳɰ����䷴Ӧ�������������ģ���������ͼ��
Ϊ�������ڲ�ͬ�������Һ��ʵ�ֳ��µ���ϳɰ����䷴Ӧ�������������ģ���������ͼ��

![]() ��________
��________![]() ����
����![]() ������
������![]() ��
��![]() ��Һ�д�Ч�����ã���
��Һ�д�Ч�����ã���![]() ��Һ��
��Һ��![]() ��Һ�У�
��Һ�У�![]() ��Ӧ�е�
��Ӧ�е�![]() ________
________![]() ����ǰ�ߴ��������ߴ�������һ������
����ǰ�ߴ��������ߴ�������һ������![]() ��
��
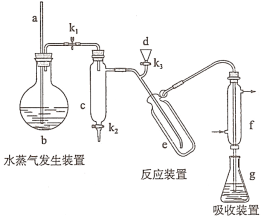
(4)![]() ʱ����һ���Ϊ2L�ĺ��ݸ����ܱ������з�����Ӧ��
ʱ����һ���Ϊ2L�ĺ��ݸ����ܱ������з�����Ӧ��![]() ���ı䷴Ӧ��һ������������������������ϵ�и����ʵ����ʵ�����ʱ��仯��������ͼ��ʾ��
���ı䷴Ӧ��һ������������������������ϵ�и����ʵ����ʵ�����ʱ��仯��������ͼ��ʾ��

��![]() �ڣ�
�ڣ�![]() ________
________![]() ��
��
��![]() ʱ�������ε�ƽ�ⳣ��
ʱ�������ε�ƽ�ⳣ��![]() Ϊ________
Ϊ________![]() ����2λ��Ч����
����2λ��Ч����![]() ��
��
�۵�����ƽ��ʱ![]() ��
��![]() �����ʵ���֮��Ϊ________��
�����ʵ���֮��Ϊ________��
�ܱȽϵ�����ƽ�ⳣ��![]() �͵�����ƽ�ⳣ��
�͵�����ƽ�ⳣ��![]() �Ĵ�С��
�Ĵ�С��![]() ________
________![]() ����
����![]() ����
����![]() ������
������![]() ��
��![]() ���жϵ�������____________________��
���жϵ�������____________________��
���𰸡�-92.4kJ/mol 600.4kJ/mol ![]() һ����
һ���� ![]()
![]()
![]()
![]() �÷�Ӧ������ӦΪ���ȷ�Ӧ����ͼ���жϵ�III���ǽ����¶ȣ�����ƽ�ⳣ������
�÷�Ӧ������ӦΪ���ȷ�Ӧ����ͼ���жϵ�III���ǽ����¶ȣ�����ƽ�ⳣ������
��������
��1��![]()
![]()
![]()
![]()
![]()
![]() ���ݸ�˹������
���ݸ�˹������![]() �ɵ�
�ɵ�![]()
![]() ��
��
��2��![]() �Ļ��
�Ļ��![]() ���ֽⷴӦ��
���ֽⷴӦ��![]() �Ļ��
�Ļ��![]() ��
��
��3����ͼ��֪��![]() ��Һ�е����ӵ������ϵͣ����
��Һ�е����ӵ������ϵͣ����![]() ��Һ��������
��Һ��������![]() �Ե����ӵĻ����Ӧ���ʱ�������͵���ʵ������أ�����ʱ�һ����
�Ե����ӵĻ����Ӧ���ʱ�������͵���ʵ������أ�����ʱ�һ����
��4��![]() ����ͼ�������֪
����ͼ�������֪![]() �ڣ����������ʵ����仯��
�ڣ����������ʵ����仯��![]() ������
������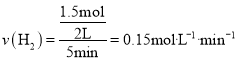 ��
��
![]() ����ͼ�������֪��I��ƽ��ʱ�����������Ͱ��������ʵ����ֱ�Ϊ1mol��3mol��2mol�����ƽ�ⳣ��Ϊ
����ͼ�������֪��I��ƽ��ʱ�����������Ͱ��������ʵ����ֱ�Ϊ1mol��3mol��2mol�����ƽ�ⳣ��Ϊ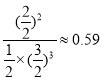 ��
��
![]() ����ͼ������ڶ��θı�������Ƿ�����˰����ӣ���˵��������������ʵ���Ϊ
����ͼ������ڶ��θı�������Ƿ�����˰����ӣ���˵��������������ʵ���Ϊ![]() ��
��
![]() ��II�ε���III�Σ�������������Ũ�ȶ��Ǵ�ԭƽ��㽵�ͣ��������ӵ�Ũ�ȴ�ԭƽ�����������ƽ��������Ӧ�����ƶ������ڸ÷�Ӧ������ӦΪ���ȷ�Ӧ�����Է�Ӧ��ϵ�����¶ȣ�ƽ�ⳣ������
��II�ε���III�Σ�������������Ũ�ȶ��Ǵ�ԭƽ��㽵�ͣ��������ӵ�Ũ�ȴ�ԭƽ�����������ƽ��������Ӧ�����ƶ������ڸ÷�Ӧ������ӦΪ���ȷ�Ӧ�����Է�Ӧ��ϵ�����¶ȣ�ƽ�ⳣ������

 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�