��Ŀ����
�������ʵ������йع�ʽ���㣨ע����2����3����4������������С�������λ��
��1��3.01��1023��NH4+��������Ϊ g��
��2��������1L 0.2 mol.L-1��������Һ�����״����HCl�����Ϊ L��
��3���ֽ�50 mL�ܶ�Ϊ1.84 g/cm3����������Ϊ98.0 %��Ũ���ᣬϡ����250 mL����ϡ�ͺ���Һ�����ʵ���Ũ��Ϊ ��
��4��19��MgCl2�к���������Ŀ ��
��5������ԭ������ȵļ���Ͱ�����NH3����������Ϊ ��
��ϰ��ϵ�д�
 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
�����Ŀ


 2NO(g)+O2(g) ��H��0��������������ȷ����
2NO(g)+O2(g) ��H��0��������������ȷ���� CH3OH��g��+H2O��g����H=-49.0kJ��mol-1��ij��ѧʵ�齫6mol CO2��8mol H2����2L���ܱ������У����H2�����ʵ�����ʱ��仯��ͼ1��ʾ��ʵ�ߣ���ͼ������a��1��6����������˼�ǣ���1minʱH2�����ʵ�����6mol��
CH3OH��g��+H2O��g����H=-49.0kJ��mol-1��ij��ѧʵ�齫6mol CO2��8mol H2����2L���ܱ������У����H2�����ʵ�����ʱ��仯��ͼ1��ʾ��ʵ�ߣ���ͼ������a��1��6����������˼�ǣ���1minʱH2�����ʵ�����6mol��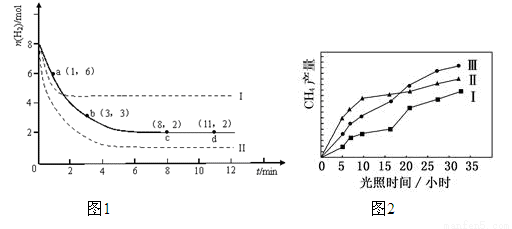
 CO2��g��+NO��g������Ӧ���������һ���ӵİٷ���Ҳ���ӵĴ�ʩ��
CO2��g��+NO��g������Ӧ���������һ���ӵİٷ���Ҳ���ӵĴ�ʩ��