��Ŀ����
����������ڱ��������ҩ����ʹЧ�����ڲ���ҡ���ͼ�ǰ�����ҵ�һ���ϳ�·�ߡ� 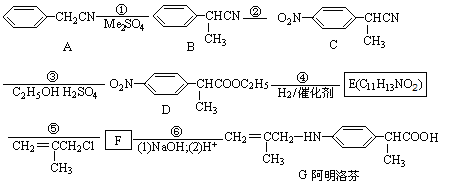
��1����Ӧ��Ϊȡ����Ӧ���ò���Ӧ��Me2SO4�еġ�Me����ʾ ��D�й����ŵ������� ��
��2��д��E�Ľṹ��ʽ�� ��
��3��G�ܷ����ķ�Ӧ������ ������ĸ��ţ�
��4��д��F��NaOH��Һ�з�Ӧ�Ļ�ѧ����ʽ�� ��
��5��д��������������������B��ͬ���칹�壺 ��
��ֻ����һ�������ȱ������ķ����廯����ں˴Ź������������ĸ������B������ͬ���칹�����١�

��1����Ӧ��Ϊȡ����Ӧ���ò���Ӧ��Me2SO4�еġ�Me����ʾ ��D�й����ŵ������� ��
��2��д��E�Ľṹ��ʽ�� ��
��3��G�ܷ����ķ�Ӧ������ ������ĸ��ţ�
| A��ȡ����Ӧ | B��������Ӧ | C���Ӿ۷�Ӧ | D����ԭ��Ӧ E���ӳɷ�Ӧ |
��5��д��������������������B��ͬ���칹�壺 ��
��ֻ����һ�������ȱ������ķ����廯����ں˴Ź������������ĸ������B������ͬ���칹�����١�
��16�֣�
��1����CH3���������2�֣�������������2�֣�
��2��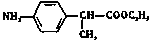 ��2�֣�
��2�֣�
��3��ABCDE��2�֣�
��4��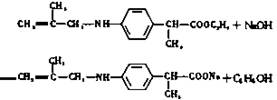 ��2�֣�
��2�֣�
��5��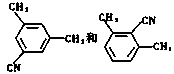 ��2�֣�
��2�֣�
��1����CH3���������2�֣�������������2�֣�
��2��
 ��2�֣�
��2�֣���3��ABCDE��2�֣�
��4��
 ��2�֣�
��2�֣���5��
 ��2�֣�
��2�֣����������
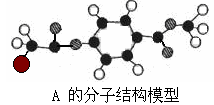
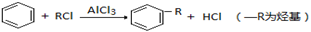
��1������A��B�ķ��Ӳ�𣬿��Կ�����һ����CH3����������Ƴ�MeΪ��CH3���������
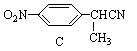 ���������������
�к�����������������2������D��E�ķ���ʽ�ܵ͢ķ�Ӧ��������֪Ϊ-NO2�Ļ�ԭ��Ӧ����֪EΪ��

��3��G�ܷ���A�������Ȼ��������ϵ�ȡ����Ӧ�� B���Ȼ���������Ӧ�� C��̼̼˫���ļӾ۷�Ӧ��D��̼̼˫���������������Ļ�ԭ��Ӧ ��E��̼̼˫���������ļӳɷ�Ӧ��
��4��
 ��
��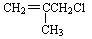 ����ȡ����Ӧ�ĵ�
����ȡ����Ӧ�ĵ�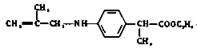 ��������������Һ����������ˮ�⡣
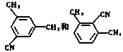
��������������Һ����������ˮ�⡣��5��������Ϣ������Ҫ�и߶ȵĶԳ��ԣ���д��
 ��
��
��ϰ��ϵ�д�
 ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д�
�����Ŀ
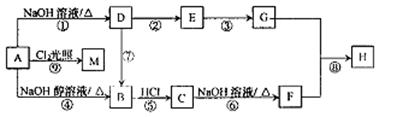
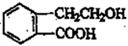 ������ͨ����ͬ�Ļ�ѧ��Ӧ�ֱ��Ƶ�B��C��D��E�������ʡ�
������ͨ����ͬ�Ļ�ѧ��Ӧ�ֱ��Ƶ�B��C��D��E�������ʡ�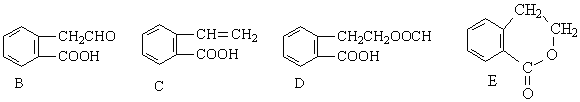
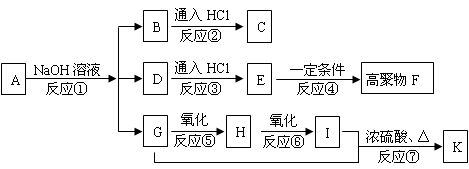
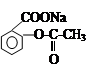 ת���
ת��� �ķ�����
�ķ�����

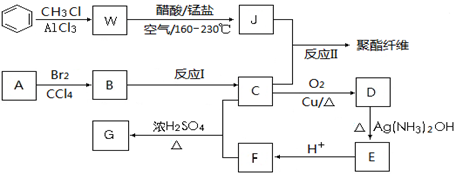

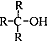 ������������ȩ��ͪ��
������������ȩ��ͪ��