��Ŀ����
�й��ܡ�����������������ʼ��±���
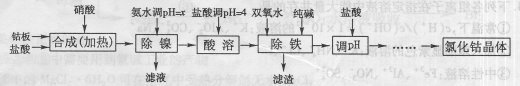
�Ȼ��ܿ����ڻ�ѧ��Ӧ����������߸ɼ�����ʪָʾ����ơ����ĭ�ȶ������մ���ɫ������������īˮ�ȣ��ý����ܰ壨������Fe��Ni���Ʊ��Ȼ��ܵĹ����������£�
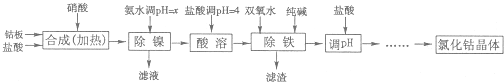
�������ᷴӦ������������������ſ��ܽ���ʵ��������
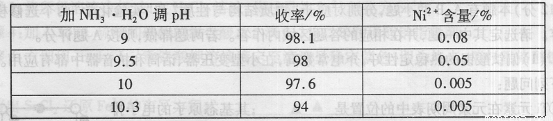
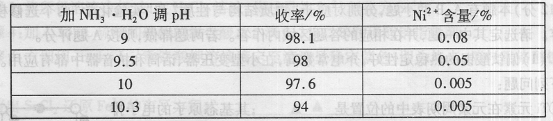
��1���������������У�NH3?H2O�����Է�Ӧ���ʵ�Ӱ�죬���±���
�ӱ��п�֪x=
��2����������������������һ����ʱ������ɣ�����������в���Co��OH��3���ɣ���д����Ӧ�Ļ�ѧ����ʽ
��3���������������м���˫��ˮ������Ӧ�����ӷ���ʽ��
��4���������������м���Ĵ���������
��5���ڡ���pH�������У��������������
��6����֪25��ʱ��Ksp[Fe��OH��3]=4.0��10-38������¶��·�ӦFe3++3H2O?Fe��OH��3+3H+��ƽ�ⳣ��Ϊ
| ��ѧʽ | ������ȫʱ��pH | �������� | ||
| Co��OH��2 | 9.4 | Co+2HCl=CoCl2+H2�� Co2++2NH3?H2O=Co��OH��2��+2NH
Ni+2HCl=NiCl2+H2�� Co2++2H2O?Co��OH��2+2H+ Ni2++6NH3?H2O=[Ni��NH3��6]2++6H2O | ||
| Fe��OH��2 | 9.6 | |||
| Fe��OH��3 | 3.7 |

�������ᷴӦ������������������ſ��ܽ���ʵ��������
��1���������������У�NH3?H2O�����Է�Ӧ���ʵ�Ӱ�죬���±���
| ��NH3?H2O��PH | ����/% | Ni2+����/% |
| 9 | 98.1 | 0.08 |
| 9.5 | 98 | 0.05 |
| 10 | 97.6 | 0.005 |
| 10.3 | 94 | 0.005 |
10
10
ʱ������Ч����ã���2����������������������һ����ʱ������ɣ�����������в���Co��OH��3���ɣ���д����Ӧ�Ļ�ѧ����ʽ
4Co��OH��2+O2+2H2O=4Co��OH��3
4Co��OH��2+O2+2H2O=4Co��OH��3
����3���������������м���˫��ˮ������Ӧ�����ӷ���ʽ��
2Fe2++H2O2+2H+=2Fe3++2H2O
2Fe2++H2O2+2H+=2Fe3++2H2O
����4���������������м���Ĵ���������
ʹ��������ת��Ϊ����������������ȥ
ʹ��������ת��Ϊ����������������ȥ
����5���ڡ���pH�������У��������������
��ֹCo2+ˮ��
��ֹCo2+ˮ��
����6����֪25��ʱ��Ksp[Fe��OH��3]=4.0��10-38������¶��·�ӦFe3++3H2O?Fe��OH��3+3H+��ƽ�ⳣ��Ϊ
2.5��10-5
2.5��10-5
����������1�����ݱ��е����ݽ��ʵ�����ش��жϣ�
��2���������״������۱����������������ۣ�
��3��˫��ˮ���������ԣ����Խ�������������Ϊ�����ۣ�
��4�����봿��̼���ƿ��Ժ��ᷢ����Ӧ��������Һ��pH�����ã�
��5�������������������ӣ��ܷ���ˮ�⣬��ʾ���ԣ�
��6�����ݻ�ѧƽ�ⳣ������ʽ��ϳ����ܽ��ܶȻ�������м��㣮
��2���������״������۱����������������ۣ�
��3��˫��ˮ���������ԣ����Խ�������������Ϊ�����ۣ�
��4�����봿��̼���ƿ��Ժ��ᷢ����Ӧ��������Һ��pH�����ã�
��5�������������������ӣ��ܷ���ˮ�⣬��ʾ���ԣ�
��6�����ݻ�ѧƽ�ⳣ������ʽ��ϳ����ܽ��ܶȻ�������м��㣮
����⣺��1�����ݱ��е�����֪������pH����10��ʱ��������ߣ������ӵĺ�����С������Ч����ã��ʴ�Ϊ��10��
��2���������״����������ױ����������������ۣ������Ļ�ѧ����ʽΪ��4Co��OH��2+O2+2H2O=4Co��OH��3���ʴ�Ϊ��4Co��OH��2+O2+2H2O=4Co��OH��3��
��3��˫��ˮ���������ԣ����Խ�������������Ϊ�����ۣ������Ļ�ѧ��ӦΪ��2Fe2++H2O2+2H+=2Fe3++2H2O���ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��4�����봿��̼���ƿ��Ժ��ᷢ����Ӧ�����ʵ��Ļ����£��������������γɳ�������ȥ�����Լ���Ĵ���������������Һ��pH�����ã��ʴ�Ϊ��ʹ��������ת��Ϊ����������������ȥ��
��5�������������������ӣ��ܷ���ˮ�⣬��ʾ���ԣ�����������Է�ֹCo2+ˮ�⣬�ʴ�Ϊ����ֹCo2+ˮ�⣻
��6��Ksp[Fe��OH��3]=c��Fe3+����c3��OH-��=4.0��10-38��c��H+��=
����ӦFe3++3H2O?Fe��OH��3+3H+��ƽ�ⳣ��K=
�T
=2.5��10-5���ʴ�Ϊ��2.5��10-5��
��2���������״����������ױ����������������ۣ������Ļ�ѧ����ʽΪ��4Co��OH��2+O2+2H2O=4Co��OH��3���ʴ�Ϊ��4Co��OH��2+O2+2H2O=4Co��OH��3��
��3��˫��ˮ���������ԣ����Խ�������������Ϊ�����ۣ������Ļ�ѧ��ӦΪ��2Fe2++H2O2+2H+=2Fe3++2H2O���ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��4�����봿��̼���ƿ��Ժ��ᷢ����Ӧ�����ʵ��Ļ����£��������������γɳ�������ȥ�����Լ���Ĵ���������������Һ��pH�����ã��ʴ�Ϊ��ʹ��������ת��Ϊ����������������ȥ��
��5�������������������ӣ��ܷ���ˮ�⣬��ʾ���ԣ�����������Է�ֹCo2+ˮ�⣬�ʴ�Ϊ����ֹCo2+ˮ�⣻
��6��Ksp[Fe��OH��3]=c��Fe3+����c3��OH-��=4.0��10-38��c��H+��=
| 10-14 |
| c(OH-) |
| c(H+)3 |
| c(Fe3+) |
| 10-42 |
| c(Fe3+)?c(OH-)3 |
=2.5��10-5���ʴ�Ϊ��2.5��10-5��
������������һ��ʵ��̽���⣬�ܽϺõĿ���ѧ�������ͽ�������������ע��ʵ�鷽�������ԭ���Ͳ������ù�ϵʽ���㣬�������е�֪ʶ������ʼ䷴Ӧ��ʵ������������ʳɷֵ��ƶϣ��ǽ��Ĺؼ���ƽʱע������ʵ�Ļ���֪ʶ�����Ӧ��֪ʶ��������������������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ


 Fe��OH��3+3H+��ƽ�ⳣ��Ϊ
��
Fe��OH��3+3H+��ƽ�ⳣ��Ϊ
��
 Fe��OH��3+3H+��ƽ�ⳣ��Ϊ ��
Fe��OH��3+3H+��ƽ�ⳣ��Ϊ ��