��Ŀ����
��10�֣�������H��һ�����ϣ������ڽ����У���������·�ߺϳɣ�
��֪��(1)
��B��C��Ϊһ�ȴ�����D��֧����
�ش��������⣺
��11.2L����״��������A�������г��ȼ�տ��Բ���88g CO2��45g H2O��A�ķ���ʽ�� ��
���ڴ���������1 mol F��2 mol H2��Ӧ������3��������1��������F�Ľṹ��ʽ�� ��
�Ƿ�Ӧ�ٵķ�Ӧ������ ��
�ȷ�Ӧ�ڵĻ�ѧ����ʽΪ ��
��д��������G������ͬ�����ŵ�G�ķ�����ͬ���칹��Ľṹ��ʽ��
��C4H10��2�֣� ��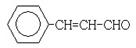 ��2�֣� ����ȥ��Ӧ
��2�֣� ����ȥ��Ӧ
��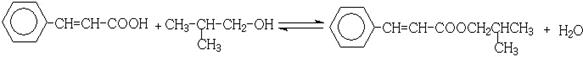 ��2�֣�
��2�֣�
�� ��
�� ��
�� ��
��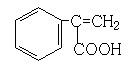 ��3�֣�
��3�֣�
���������������1��88gCO2Ϊ2mol��45gH2OΪ2.5mol����״����11.2L��������ʵ���Ϊ0.5mol��������A�к�̼ԭ��Ϊ4,Hԭ����Ϊ10����ѧʽΪC4H10��
��2��F������Cu(OH)2��Ӧ����ӦΪȩ������H2֮��Ϊ1��2�ӳɣ���Ӧ����̼̼˫���������ɵIJ���3-����-1-����������F�Ľṹ��ʽΪ ��
��
��3����Ӧ��Ϊ±�����ڴ���Һ�е���ȥ��Ӧ��
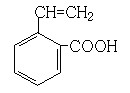
��4��F�����Ƶ�Cu(OH)2���������ᣬD��EΪȻ������Ϣ��ͬ������������ȿɲ��ѵó�E�ĽṹΪ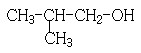 ��E��G��Ũ���������¿��Է���������Ӧ��
��E��G��Ũ���������¿��Է���������Ӧ��
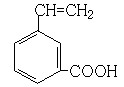
��5��G�к��й�������̼̼˫�����Ȼ������Խ�����������Ӧ��λ�ñ任���ó��䷼�����ͬ���칹�塣
���㣺�����л��ﻯѧʽ���л���Ӧ���͡��ṹ��ʽ��ͬ���칹����й��ж��Լ�����ʽ����д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����������У��ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ���������������ͷ�ɢ˼ά����������Ĺؼ�����ȷ���ֹ����Žṹ�����ʣ��ر��ǹ�����֮����ת����ϵ��Ȼ��������������⡢����������ɣ�����������ѧ���Ĵ���˼ά������Ӧ��������

 ������H��һ�����ϣ������ڽ����У���������·�ߺϳɣ�
������H��һ�����ϣ������ڽ����У���������·�ߺϳɣ�


 |
 ��֪��
��֪��
 �ش��������⣺
�ش��������⣺ ������1��11.2L����״��������A�������г��ȼ�տ��Բ���88 g CO2��45 g H2O��
������1��11.2L����״��������A�������г��ȼ�տ��Բ���88 g CO2��45 g H2O�� A�ķ���ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
A�ķ���ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ� ������2��B��C��Ϊһ�ȴ��������ǵ����ƣ�ϵͳ�������ֱ�Ϊ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
������2��B��C��Ϊһ�ȴ��������ǵ����ƣ�ϵͳ�������ֱ�Ϊ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ� ��3���ڴ���������1 mol F��2 mol H2��Ӧ������3��������1��������F�Ľṹ��ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣ�
��3���ڴ���������1 mol F��2 mol H2��Ӧ������3��������1��������F�Ľṹ��ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣ� ��4����Ӧ�ٵķ�Ӧ�����ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
��4����Ӧ�ٵķ�Ӧ�����ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ� ��5����Ӧ�ڵĻ�ѧ����ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
��5����Ӧ�ڵĻ�ѧ����ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ� ��6��д��������G������ͬ�����ŵ�G�ķ�����ͬ���칹��Ľṹ��ʽ��
��6��д��������G������ͬ�����ŵ�G�ķ�����ͬ���칹��Ľṹ��ʽ��



 ��
�� ��
�� ��
��






 ��
�� ��
�� ��
��

