��Ŀ����
��12�֣������к��зḻ�ĵ⡣Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
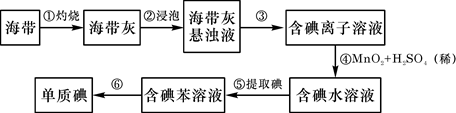
����д���пհף�
(1) ��������պ���ʱ������Ҫ���żܡ��ƾ����⣬����Ҫ�õ���ʵ��������
��������������ѡ��������������ñ����ĸ��д�ڿհ״�����
(2) ����۵�ʵ����������� ;�����Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ����ò����ʵ����������� ��
(3) ����ܷ�Ӧ�����ӷ���ʽ�� ��
(4) ������У�ijѧ��ѡ���ñ�����ȡ��������� ��
(5) �����һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ����� ��

����д���пհף�
(1) ��������պ���ʱ������Ҫ���żܡ��ƾ����⣬����Ҫ�õ���ʵ��������
��������������ѡ��������������ñ����ĸ��д�ڿհ״�����
| A���ձ� | B������ | C�������� | D�������� E.������ |
(3) ����ܷ�Ӧ�����ӷ���ʽ�� ��
(4) ������У�ijѧ��ѡ���ñ�����ȡ��������� ��
(5) �����һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ����� ��
�� 12�֣���ÿ��2�֣� (1)B D (2)���� ���� (3)2I-+MnO2+4H+=Mn2++I2+2H2O
(4)����ˮ��������;���ڱ��е��ܽ�ȱ���ˮ�д�
(5)ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ;�۲��Ƿ������ɫ(���������˵�����е��ʵ�)
(4)����ˮ��������;���ڱ��е��ܽ�ȱ���ˮ�д�
(5)ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ;�۲��Ƿ������ɫ(���������˵�����е��ʵ�)
��1�����������Ӧ������������ɣ���������Ҫ���������ǵ����ż��ϣ���ѡBD��
��2�������ҵ�����Һ����Ҫͨ�����˳�ȥ����ͱ����ܣ�Ҫ����õ���ͱ����������ö��߷е�IJ�࣬ͨ������õ���
��3���������̾��������ԣ������������ӣ����ɵ��ʵ⣬����ʽΪ)2I-+MnO2+4H+=Mn2++I2+2H2O��
��4������ˮ�������ܣ��ҵ��ڱ��е��ܽ�ȱ���ˮ�д����Կ�������ȡ����
��5����Ϊ���ۺ͵��ܷ�����ɫ��Ӧ���Ӿݴ˿��Լ��飬��ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ;�۲��Ƿ������ɫ(���������˵�����е��ʵ�)��
��2�������ҵ�����Һ����Ҫͨ�����˳�ȥ����ͱ����ܣ�Ҫ����õ���ͱ����������ö��߷е�IJ�࣬ͨ������õ���
��3���������̾��������ԣ������������ӣ����ɵ��ʵ⣬����ʽΪ)2I-+MnO2+4H+=Mn2++I2+2H2O��
��4������ˮ�������ܣ��ҵ��ڱ��е��ܽ�ȱ���ˮ�д����Կ�������ȡ����
��5����Ϊ���ۺ͵��ܷ�����ɫ��Ӧ���Ӿݴ˿��Լ��飬��ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ;�۲��Ƿ������ɫ(���������˵�����е��ʵ�)��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ