��Ŀ����
A.B.C.D����Ԫ�غ˵������С��18��BԪ���ǵؿ��к�������Ԫ�أ�A.CԪ�ص�ԭ��������������ȣ�C.DԪ��ԭ�ӵ��Ӳ�����ͬ��Aԭ���������������ڵ��Ӳ�����Cԭ��������������K���ϵ�������һ�롣DԪ��ԭ�����������Ӳ㣬D-���ӵ��Ӳ�ṹ���ԭ�ӵ��Ӳ�ṹ��ͬ��������Ԫ�ص�����Ϊ��
��1��A B C D ��
��2��Aԭ�ӡ�C+���ӵĽṹʾ��ͼΪ �� ��
��3��Bԭ�ӡ�D-���ӵĵ���ʽΪ �� ��
��4��A������B���ʵĻ�ѧ����ʽΪ ��
��1��A B C D ��
��2��Aԭ�ӡ�C+���ӵĽṹʾ��ͼΪ �� ��
��3��Bԭ�ӡ�D-���ӵĵ���ʽΪ �� ��
��4��A������B���ʵĻ�ѧ����ʽΪ ��
��1��H O Na Cl
��2��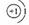
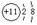
��3��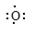

��4��2H2+O2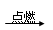 2H2O
2H2O
��2��
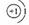
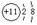
��3��
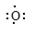

��4��2H2+O2
 2H2O
2H2O�����������1��BԪ���ǵؿ��к�������Ԫ��ΪOԪ�أ�DԪ��ԭ�����������Ӳ㣬D-���ӵ��Ӳ�ṹ���ԭ�ӵ��Ӳ�ṹ��ͬ��DΪCl ��Cԭ��������������K���ϵ�������һ����C.DԪ��ԭ�ӵ��Ӳ�����ͬ��CΪNa��Aԭ���������������ڵ��Ӳ�����A.CԪ�ص�ԭ��������������ȣ���AΪH����2��
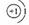
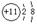 ��3��
��3��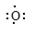
 ��4��2H2+O2
��4��2H2+O2 2H2O
2H2O
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
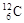 ��
��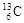 ��
��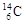 ��˵����ȷ����
��˵����ȷ����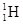 ��
��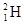 ��
��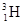 ��˵����ȷ���ǣ� ��
��˵����ȷ���ǣ� ��