��Ŀ����
 ʵ����
ʵ����
�������й�������ʹ�ã���ʵ�ֵ���______������ţ�
A����������ƽ��ȡ11.70gʳ��
B������Ͳ��ȡ12.36ml����
C������ʽ�ζ�����ȡ21.20ml 0.10mol/L H2SO4��Һ
D����200ml����ƿ����500ml 0.1mol/L NaCl��Һ
���������ʵ���Ũ��Ϊa mol/L�ı�����ȥ�ⶨV mL NaOH��Һ�����ʵ���Ũ�ȣ�����д���пհף�
��1����ʽ�ζ���������ˮϴ����Ӧ�ý��еIJ�����______��
��2����ͼ����ʽ�ζ�����Һ���ڵζ�ǰ��Ķ�������c ��NaOH��=______��
��3�����ڵζ�ǰ�ζ��ܼ��첿���������ݣ��ζ���ζ��ܼ��첿��������ʧ����ⶨ��NaOH���ʵ���Ũ�Ȼ�ƫ______��
�⣺��A��������ƽ��ȷ��0.1�����Ƴ�11.70gʳ�Σ���A���� B����Ͳ��ȷ��0.1������ȡ12.36ml���ᣬ��B����
C���ζ��ܾ�ȷ��0.01������ȡ21.20ml 0.10mol/L H2SO4��Һ����C��ȷ��
D������500ml 0.1mol/L NaCl��ҺӦ��500ml����ƿ����D����
��ѡC��
��1���ζ���ʹ��ǰӦ�ô���Һ��ϴ���ʴ�Ϊ���ñ�������2���к͵ζ�����ͼ�����ʵ�����ȣ���V2-V1��a=c ��NaOH��V���ʴ�Ϊ��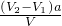 ��
��
��3���ζ�ǰ��ʽ�ζ��ܼ��첿�������ݣ��ζ�����첿�ֳ�����Һ���ᵼ���������ƫ�������ƫ�ʴ�Ϊ����
��������A��������ƽ��ȷ��0.1��
B����Ͳ��ȷ��0.1��
C���ζ��ܾ�ȷ��0.01��
D������ƿֻ�����ù̶��������Һ��
��1���ζ���ʹ��ǰӦ��ϴ�� ��2���к͵ζ�����ͼ�����ʵ�����ȣ�
��3�����ݹ�ϵʽc�����⣩��V�����⣩=c��������V��������
���������⿼���к͵ζ����ѶȲ���ע���״�������������
C���ζ��ܾ�ȷ��0.01������ȡ21.20ml 0.10mol/L H2SO4��Һ����C��ȷ��
D������500ml 0.1mol/L NaCl��ҺӦ��500ml����ƿ����D����
��ѡC��
��1���ζ���ʹ��ǰӦ�ô���Һ��ϴ���ʴ�Ϊ���ñ�������2���к͵ζ�����ͼ�����ʵ�����ȣ���V2-V1��a=c ��NaOH��V���ʴ�Ϊ��
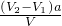 ��
����3���ζ�ǰ��ʽ�ζ��ܼ��첿�������ݣ��ζ�����첿�ֳ�����Һ���ᵼ���������ƫ�������ƫ�ʴ�Ϊ����
��������A��������ƽ��ȷ��0.1��
B����Ͳ��ȷ��0.1��
C���ζ��ܾ�ȷ��0.01��
D������ƿֻ�����ù̶��������Һ��
��1���ζ���ʹ��ǰӦ��ϴ�� ��2���к͵ζ�����ͼ�����ʵ�����ȣ�
��3�����ݹ�ϵʽc�����⣩��V�����⣩=c��������V��������
���������⿼���к͵ζ����ѶȲ���ע���״�������������

��ϰ��ϵ�д�
 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
�����Ŀ
 ʵ����
ʵ����
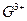 ������Arԭ�ӵĵ��Ӳ�ṹ��ͬ����ش�:
������Arԭ�ӵĵ��Ӳ�ṹ��ͬ����ش�: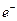 �����ȶ��ṹ
�����ȶ��ṹ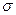 �� h��
�� h��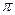 ��
��
 ����ˮ���ձ��У��ò��������Ͻ��裬��ʹ�����������ø��ձ��е�������ˮ��a�ڲ����IJ����ϴ���ձ��У����ˣ���������ˮϴ�ӣ��õ��ֲ�Ʒ��
����ˮ���ձ��У��ò��������Ͻ��裬��ʹ�����������ø��ձ��е�������ˮ��a�ڲ����IJ����ϴ���ձ��У����ˣ���������ˮϴ�ӣ��õ��ֲ�Ʒ��
 ʵ����
ʵ����