��Ŀ����
(14��)�Ķ���������¸������ݣ�
SO2�dz����Ĵ�����Ⱦ��֮һ���ҹ��涨������SO2�������ó���0.02 mg/L��
(1)�����ǽ�ˮ������(pHС��5.6)��ͳ�ƣ�����ط���������2.1(ʳ��pH��3)�������������������Ǵ����е�SO2�͵�����������ǵ���Ҫ��Դ��ú��ʯ�͵�ȼ�գ�ȫ����ÿ���ŷ�1.5�ڶֵ�SO2��
��SO2���ڿ������ܹ��յ��������ö�����������������ˮ�γ����������ꡣ��д����������ѧ��Ӧ�ķ���ʽ��________________ �� ��
�������ŷŵ�β�������᳧�ͻ��ʳ��ķ����ж����е��������ȫ����ÿ���ŷ���ԼΪ5��107 kg��NO2����ˮ���� ________��NO��
������ɵ��µ�Σ����________(�����)��
A����ʴ������ B��������ľ��ή C����ɺ����ֺ� D���������
��Ϊ�˼���������γɣ��������SO2���ŷ�������ȼ���е��������________���Է����еĵ�����������________���ա�
(2)ij��ѧ��ȤС��ѡ����ͼʵ��װ�ã��ⶨ��ҵԭ����(��SO2��N2��O2)��SO2�ĺ���(��֪��������H2SO3������H2SO4)��
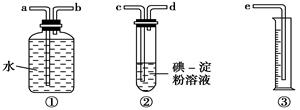
����ԭ��������������ʱ������װ�����ӵ�˳���ǣ�ԭ������________ (����ĸ�ͼ�ͷ��ʾ)��
��װ�â��з�����Ӧ�����ӷ���ʽΪ�� ��
��װ�â��г���________����ʱ������ֹͣͨ����
������Ϊ�����Լ��У��������������Թ��еĵ�ĵ�����Һ����________��
A������KMnO4��Һ B��NaOH��Һ C����ˮ D����ˮ
SO2�dz����Ĵ�����Ⱦ��֮һ���ҹ��涨������SO2�������ó���0.02 mg/L��
(1)�����ǽ�ˮ������(pHС��5.6)��ͳ�ƣ�����ط���������2.1(ʳ��pH��3)�������������������Ǵ����е�SO2�͵�����������ǵ���Ҫ��Դ��ú��ʯ�͵�ȼ�գ�ȫ����ÿ���ŷ�1.5�ڶֵ�SO2��
��SO2���ڿ������ܹ��յ��������ö�����������������ˮ�γ����������ꡣ��д����������ѧ��Ӧ�ķ���ʽ��________________ �� ��
�������ŷŵ�β�������᳧�ͻ��ʳ��ķ����ж����е��������ȫ����ÿ���ŷ���ԼΪ5��107 kg��NO2����ˮ���� ________��NO��
������ɵ��µ�Σ����________(�����)��
A����ʴ������ B��������ľ��ή C����ɺ����ֺ� D���������
��Ϊ�˼���������γɣ��������SO2���ŷ�������ȼ���е��������________���Է����еĵ�����������________���ա�
(2)ij��ѧ��ȤС��ѡ����ͼʵ��װ�ã��ⶨ��ҵԭ����(��SO2��N2��O2)��SO2�ĺ���(��֪��������H2SO3������H2SO4)��

����ԭ��������������ʱ������װ�����ӵ�˳���ǣ�ԭ������________ (����ĸ�ͼ�ͷ��ʾ)��
��װ�â��з�����Ӧ�����ӷ���ʽΪ�� ��
��װ�â��г���________����ʱ������ֹͣͨ����
������Ϊ�����Լ��У��������������Թ��еĵ�ĵ�����Һ����________��
A������KMnO4��Һ B��NaOH��Һ C����ˮ D����ˮ
��1����2SO2+O2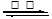 2SO3����1�֣�SO3+H2O
2SO3����1�֣�SO3+H2O H2SO4��1�֣�
H2SO4��1�֣�
�����ᣨHNO3����1�֣� ��A B D��1�֣�������ţ� ������1�֣���NaOH��Һ��1�֣�
��2����ԭ������c��d��b��a��e��2�֣�������ĸ�ͼ�ͷ��ʾ��
��SO2+I2+2H2O=4H++2I-+SO42-��2�֣���ɫ��ȥ��1�֣���AC��1�֣�
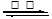 2SO3����1�֣�SO3+H2O
2SO3����1�֣�SO3+H2O H2SO4��1�֣�
H2SO4��1�֣������ᣨHNO3����1�֣� ��A B D��1�֣�������ţ� ������1�֣���NaOH��Һ��1�֣�
��2����ԭ������c��d��b��a��e��2�֣�������ĸ�ͼ�ͷ��ʾ��
��SO2+I2+2H2O=4H++2I-+SO42-��2�֣���ɫ��ȥ��1�֣���AC��1�֣�
���黯ѧ�����������ɼ����������ȡ�
��1����SO2���л�ԭ�ԣ�����������SO3��SO3����ˮ���������ᣬ����ʽΪ
2SO2+O2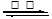 2SO3�� SO3+H2O
2SO3�� SO3+H2O H2SO4��
H2SO4��
��NO2����ˮ���������NO��
������ͺ����ֺ��أ���������ȷ�ģ���ѡABD��
��Ҫ����SO2���ŷţ���������������������������������������Һ���գ��Է�ֹ��Ⱦ������
��2���������͵���������ˮ������ͨ����ˮ�������������������ȷ��˳��Ϊԭ������c��d��b��a��e��
�ڸ��ݷ�Ӧ����������֪����Ӧ�����ӷ���ʽΪSO2+I2+2H2O=4H++2I-+SO42-�����ڵ�����������ɫ�����Ե���Һ��ɫ��ȥʱ����ֹͣͨ����
���������ʶ�������SO2�����������ƺͰ�ˮ���жϷ�Ӧ�յ㣬��˳����������Һ����ˮ��������ɫ�ģ������ж��յ㡣��ѡAC��
��1����SO2���л�ԭ�ԣ�����������SO3��SO3����ˮ���������ᣬ����ʽΪ
2SO2+O2
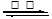 2SO3�� SO3+H2O
2SO3�� SO3+H2O H2SO4��
H2SO4����NO2����ˮ���������NO��
������ͺ����ֺ��أ���������ȷ�ģ���ѡABD��
��Ҫ����SO2���ŷţ���������������������������������������Һ���գ��Է�ֹ��Ⱦ������
��2���������͵���������ˮ������ͨ����ˮ�������������������ȷ��˳��Ϊԭ������c��d��b��a��e��
�ڸ��ݷ�Ӧ����������֪����Ӧ�����ӷ���ʽΪSO2+I2+2H2O=4H++2I-+SO42-�����ڵ�����������ɫ�����Ե���Һ��ɫ��ȥʱ����ֹͣͨ����
���������ʶ�������SO2�����������ƺͰ�ˮ���жϷ�Ӧ�յ㣬��˳����������Һ����ˮ��������ɫ�ģ������ж��յ㡣��ѡAC��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ