��Ŀ����
�����й�ʵ��˵����ȷ���ǣ�������
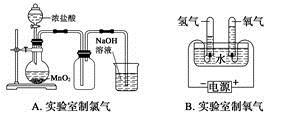
| A��ʵ�����Ʊ���ϩʱ������������ʹ��ˮ��ɫ��˵������ϩ���� |
| B����Һ�塢���ۺͱ���ϼ��ȼ����Ƶ��屽����ȥ�屽�к���ɫ���壬����ϡNaOH��Һ����ϴ�ӣ����÷�Һ©����Һ |
| C����һ֧�Թ��е���10�������飬�ټ���1mL5%��NaOH��Һ�����Ⱥ�μ���������Һ���ɹ۲쵽��dz��ɫ�廯���������� |
| D����ȡ������ʱ��ӦȡŨH2SO42mL������1.5mLŨHNO3���ٵ��뱽Լ1mL��Ȼ�����ˮԡ�м��ȣ��¶ȼ�Ӧ���ڻ��Һ�� |
A�����ڷ�Ӧ���и���������������ɣ����������ܹ�����ˮ��Ӧʹ��ˮ��ɫ�����Ա����Ƚ������������ʳ�ȥ�ټ�����ϩ����A����
B�������屽������������Һ��������Ӧ���������ܹ�������Ӧ����Ӧ����Һ�ֲ㣬���Կ���ϡNaOH��Һ����ϴ�ӣ����÷�Һ©����Һ����B��ȷ��
C��ֻ�������������²��������廯�����������Ⱥ������ϡ�����к�����������Һ��Ȼ��μ���������Һ���ɹ۲쵽��dz��ɫ�廯���������ɣ���C����
D��Ӧ���ȼ��뱽���ټ���Ũ���ᣬ�μ�˳��ߵ���������Σ�գ���D����
��ѡ��B��
B�������屽������������Һ��������Ӧ���������ܹ�������Ӧ����Ӧ����Һ�ֲ㣬���Կ���ϡNaOH��Һ����ϴ�ӣ����÷�Һ©����Һ����B��ȷ��
C��ֻ�������������²��������廯�����������Ⱥ������ϡ�����к�����������Һ��Ȼ��μ���������Һ���ɹ۲쵽��dz��ɫ�廯���������ɣ���C����
D��Ӧ���ȼ��뱽���ټ���Ũ���ᣬ�μ�˳��ߵ���������Σ�գ���D����
��ѡ��B��

��ϰ��ϵ�д�
�����Ŀ



 Ca2��+
Ca2��+