��Ŀ����
��9�֣�Ŀǰ�����ϱȽ��Ƚ��ĵ���Ƽ�������ӽ���Ĥ����
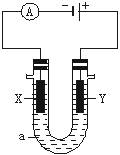
��1�����ǰ�����������SO42�������ϸߣ����������Լ���ȥSO42�����������Լ�˳�����η�����Ӧ�����ӷ���ʽΪ ��
��2���������е�ⱥ��ʳ��ˮ���ռ�����ӷ���ʽ��
______________________ __________________________________
__________________________________
����Ӧ� ��������� ���������
��3���ڵ���Ƶõ�NaOH������������һ������NaCl����˱���������ι������ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ�� ����ȴ�� ����д�������ƣ���ȥNaCl��
��4����֪NaCl��60����ܽ��Ϊ37��1 g���ֵ��60�澫�Ʊ���ʳ��ˮ1371 g����������������Һ�ܶ�Ϊ1��37 g��cm-3�����к���20gNaCl�������NaOH�����ʵ���Ũ��Ϊ__________mo1��L-1������С�����1λ����
��1�����ǰ�����������SO42�������ϸߣ����������Լ���ȥSO42�����������Լ�˳�����η�����Ӧ�����ӷ���ʽΪ ��
��2���������е�ⱥ��ʳ��ˮ���ռ�����ӷ���ʽ��
______________________
 __________________________________
__________________________________����Ӧ� ��������� ���������
��3���ڵ���Ƶõ�NaOH������������һ������NaCl����˱���������ι������ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ�� ����ȴ�� ����д�������ƣ���ȥNaCl��
��4����֪NaCl��60����ܽ��Ϊ37��1 g���ֵ��60�澫�Ʊ���ʳ��ˮ1371 g����������������Һ�ܶ�Ϊ1��37 g��cm-3�����к���20gNaCl�������NaOH�����ʵ���Ũ��Ϊ__________mo1��L-1������С�����1λ����
��1��Ba2+ + SO42�� = BaSO4�� CO32��+ Ba2+ =BaCO3�� 2H+ + CO32��= H2O+CO2����3�֣�˳��ߵ�û�֣�
��2��2Cl��+2H2O H2�� + 2OH�� + Cl2�� ��2�֣�
H2�� + 2OH�� + Cl2�� ��2�֣�
�������� ��������
��3���������� ��ÿ��1�֣���2�֣� ��4��7��1��2�֣�
��2��2Cl��+2H2O
 H2�� + 2OH�� + Cl2�� ��2�֣�
H2�� + 2OH�� + Cl2�� ��2�֣��������� ��������
��3���������� ��ÿ��1�֣���2�֣� ��4��7��1��2�֣�
��1����ȥSO42����Ҫ�Ȼ�����Һ�����������Ȼ�������Ҫ̼������Һ�������������ȥ������̼���ƣ�����йط�Ӧ�ķ���ʽ��Ba2+ + SO42�� = BaSO4����CO32��+ Ba2+ =BaCO3�� ��2H+ + CO32��= H2O+CO2����
��2�����Ե缫����ʳ��ˮʱ�������������ӷŵ����������������������ӷŵ�����������ͬʱ�ƻ�������Χˮ�ĵ���ƽ�⣬��������������������ɣ����Է�Ӧ�ķ���ʽ��2Cl��+2H2O H2�� + 2OH�� + Cl2��
H2�� + 2OH�� + Cl2��
�������� ��������
��3�������������ƺ��Ȼ��Ƶ��ܽ�����¶ȵı仯���ϴ����Կ���ͨ����������ȴ���˵ķ�����ȥ�Ȼ��ơ�
��4�����ǰ�Ȼ��Ƶ��Ȼ��Ƶ�������1371g��37.1/137.1��371g���������Ȼ�����20g�����Ե����Ȼ�����351g�����ʵ�����351g��58.5g/m��6mol����˸��ݷ�Ӧ�ķ���ʽ��֪����������������6mol����������������3mol�����Է�Ӧ�����Һ������1371g��2g/mol��3mol��71g/mol��3mol��1152g������Һ�������1152g��1.37g/ml��840.9ml�������������Ƶ�Ũ����6mol��0.841L��7.1mol/L��
��2�����Ե缫����ʳ��ˮʱ�������������ӷŵ����������������������ӷŵ�����������ͬʱ�ƻ�������Χˮ�ĵ���ƽ�⣬��������������������ɣ����Է�Ӧ�ķ���ʽ��2Cl��+2H2O
 H2�� + 2OH�� + Cl2��
H2�� + 2OH�� + Cl2�� �������� ��������
��3�������������ƺ��Ȼ��Ƶ��ܽ�����¶ȵı仯���ϴ����Կ���ͨ����������ȴ���˵ķ�����ȥ�Ȼ��ơ�
��4�����ǰ�Ȼ��Ƶ��Ȼ��Ƶ�������1371g��37.1/137.1��371g���������Ȼ�����20g�����Ե����Ȼ�����351g�����ʵ�����351g��58.5g/m��6mol����˸��ݷ�Ӧ�ķ���ʽ��֪����������������6mol����������������3mol�����Է�Ӧ�����Һ������1371g��2g/mol��3mol��71g/mol��3mol��1152g������Һ�������1152g��1.37g/ml��840.9ml�������������Ƶ�Ũ����6mol��0.841L��7.1mol/L��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ