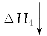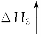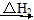ΧβΡΩΡΎ»ί
Β≥ΒΡ °ΤΏ¥σ±®Ηφ÷Η≥ωΘΚΓΑΦ”«ΩΡή‘¥Ή ‘¥ΫΎ‘ΦΚΆ…ζΧ§ΜΖΨ≥±ΘΜΛΘ§‘ω«ΩΩ…≥÷–χΖΔ’ΙΡήΝΠΘ§Φα≥÷ΫΎ‘ΦΉ ‘¥ΚΆ±ΘΜΛΜΖΨ≥ΒΡΜυ±ΨΙζ≤ΏΘ§ΖΔ’ΙΜΖ±Θ≤ζ“ΒΓΘΓ±œ¬Ν–”–ΙΊΉωΖ®≤ΜΖϊΚœ’β“Μ“Σ«σΒΡ «(ΓΓΓΓ)ΓΘ
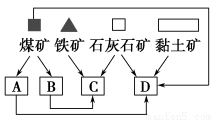
AΘ°ΫΪΟΚ“ΚΜ·ΓΔΤχΜ·Θ§ΧαΗΏ»ΦΝœΒΡ»Φ…’–߬
BΘ°Α≤ΉΑΤϊ≥ΒΈ≤Τχ¥ΏΜ·ΉΣΜ·ΉΑ÷ΟΘ§ Ι÷°ΖΔ…ζ»γœ¬Ζ¥”ΠΘΚ4COΘΪ2NO2 4CO2ΘΪN2
4CO2ΘΪN2
CΘ°‘Ύ¥σΝΠΆΤΙψ““¥ΦΤϊ”ΆΒΡΆ§ ±Θ§―–ΨΩΩΣΖΔΧΪ―τΡήΤϊ≥ΒΚΆ«β»ΦΝœΒγ≥ΊΤϊ≥Β
DΘ°‘ΎΥ°ΝΠΖΔΒγΓΔΜπΝΠΖΔΒγΓΔΚΥΡήΖΔΒγΚΆΖγΝΠΖΔΒγ÷–Θ§“Σ¥σΝΠΖΔ’ΙΜπΝΠΖΔΒγ
D
ΓΨΫβΈωΓΩA÷–ΫΪΟΚ“ΚΜ·ΚΆΤχΜ·Θ§ «Ε‘ΟΚΫχ––…νΦ”ΙΛΘ§Ρή”––ßΧαΗΏΟΚ»Φ…’≤ζ…ζΡήΝΩΒΡάϊ”Ο¬ ΘΜB÷–Θ§Τϊ≥ΒΈ≤Τχ «¥σΤχΒΡ÷Ί“ΣΈέ»ΨΈο÷°“ΜΘ§Ι Ε‘Τϊ≥ΒΈ≤ΤχΫχ––¥ΠάμΘ§ΖϊΚœΜΖΨ≥±ΘΜΛΒΡ“Σ«σΘΜC÷–ΆΤΙψ““¥ΦΤϊ”ΆΘ§”–άϊ”ΎΦθ…ΌΈέ»ΨΈοΒΡ≈≈Ζ≈ΝΩΘ§ΩΣΖΔ–¬Ε·ΝΠΤϊ≥ΒΩ…ΫΎ‘Φ≤ΜΩ…‘Ό…ζΉ ‘¥Θ§Φθ…ΌΕΰ―θΜ·ΧΦΒ»Έ¬ “ΤχΧεΒΡ≈≈Ζ≈ΘΜD÷–ΜπΝΠΖΔΒγΒΡ÷ς“Σ»ΦΝœ «ΟΚΘ§Τδ»Φ…’≤ζ…ζΒΡΕΰ―θΜ·Νρ «¥σΤχΈέ»ΨΈοΘ§Εΰ―θΜ·ΧΦΡή“ΐΤπΈ¬ “–ß”ΠΘ§ΕΦ≤Μάϊ”ΎΜΖΨ≥±ΘΜΛΘ§≤ΜΖϊΚœΜΖ±Θ“Σ«σΓΘ

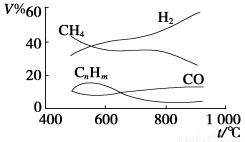
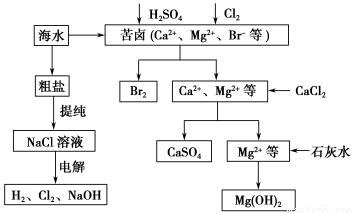
 ΜΤΗ‘ΧλΧλΝΖΩΎΥψΧβΩ®œΒΝ–¥πΑΗ
ΜΤΗ‘ΧλΧλΝΖΩΎΥψΧβΩ®œΒΝ–¥πΑΗ