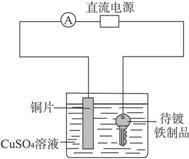
��Ŀ����
�����dz����ĵ绯ѧװ��ͼ��
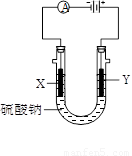
ͼ1 ͼ2 ͼ3
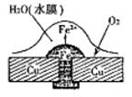
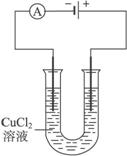
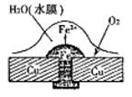
��ش��������⣺��1��ͼ1��ͭ������í�����������⣬��Ϊ ��ʴ������ʴ�Ľ����� ��ԭ��ص��ܷ�Ӧ����ʽ�� ��
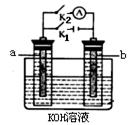
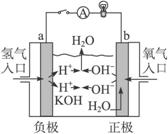
 ��2��ͼ2��a��b�Ƕ��ʯī�缫���Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��ֻ�������ڶ������ݽ��缫��Χ��b���ϵĵ缫��ӦʽΪ
��OH-��
����a��b�����ƶ���Ȼ��Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��b���ϵĵ缫��ӦʽΪ
��OH-�� ����a��b�����ƶ���
��2��ͼ2��a��b�Ƕ��ʯī�缫���Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��ֻ�������ڶ������ݽ��缫��Χ��b���ϵĵ缫��ӦʽΪ
��OH-��
����a��b�����ƶ���Ȼ��Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��b���ϵĵ缫��ӦʽΪ
��OH-�� ����a��b�����ƶ���
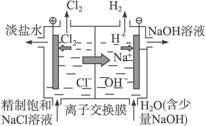
��3��ͼ3�У�X��Y��Ϊʯī�缫����U������ֱ����һ����ɫʯ����Һ����ͨ���X������______ɫ��Y������_______ɫ��
��1�� ���� Fe 2Fe+O2+2H2O=2Fe(OH)2
��2�� 2H++2e��=H2 a H2+2OH����2e��=2H2O b ��3���� ��
����������1�������������Ļ�����������Һ�����Ժ���������������ʴ������ͭ���ã���ʧȥ���ӱ���ʴ�����������ܽ���ˮ�е������õ����ӣ�������ԭ��Ӧ��
��2���Ͽ�K2���պ�K1�ɵ��أ��缫����ʯī���������������Һ���൱�ڵ��ˮ��b���Դ�ĸ����������������������ӷŵ�������������Һ�е�OH���������Ϸŵ磬����������������a���ƶ����Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��˵����ʱ����ԭ��أ���b��Χ���������ڣ��������������Һ�е��������������ƶ������������ƶ���
��3��ʯī�缫�����������Һ���൱�ڵ��ˮ��Y���Դ����������������������Һ�е�OH���ŵ磬����������X����������Һ�е������ӷŵ��������������X��Χ��Һ�Լ��ԣ�Y��Χ��Һ�����ԡ�


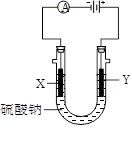


 ��2��ͼ2��a��b�Ƕ��ʯī�缫���Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��ֻ�������ڶ������ݽ��缫��Χ��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���Ȼ��Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���
��2��ͼ2��a��b�Ƕ��ʯī�缫���Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��ֻ�������ڶ������ݽ��缫��Χ��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���Ȼ��Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת��b���ϵĵ缫��ӦʽΪ ��OH-�� ����a��b�����ƶ���