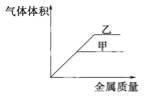
��Ŀ����
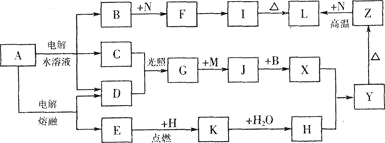
����Ŀ��ͼ����ĸ�����������ʾ�Ϊ��ѧ��ѧ�������ʡ�����A���ճ������в���ȱ�ٵ����ʣ�Ҳ�ǻ��������ϵ���Ҫԭ�ϣ�������C��D��HΪ���嵥�ʡ�����E��M��NΪ������N�ǵؿ��к������Ľ���Ԫ�ء�Y�Ǻ��ɫ��������Щ������һ�������´�������ת����ϵ��������Щ��Ӧ����������Ѿ���ȥ���Իش��������⣺
(1)Z��L��Ӧ��������______________��
(2)K�ĵ���ʽΪ______________��
(3)д��B��F�����ӷ���ʽ______________��
(4)д��K��CO2��Ӧ�Ļ�ѧ����ʽ______________��
(5)Y��NaClO��B�Ļ����Һ���ã����Ʊ���ɫˮ������(Na2MO4)��һ�ַ�������д���÷�Ӧ�����ӷ���ʽ__________________��
���𰸡�
(1)���ȷ�Ӧ��(2)![]()
(3)2Al+2OH-+2H2O��2AlO2-+3H2��
(4)2Na2O2+2CO2��2Na2CO3+O2��
(5)2Fe(OH)3+3ClO-+4OH-��2FeO42-+3Cl-+5H2O
��������
���������������C��D��HΪ���嵥�ʣ�����E��M��NΪ������N�ǵؿ��к������Ľ���Ԫ�أ���֪NΪ����Y�Ǻ��ɫ������YΪ������������ZΪFe2O3��LΪAl2O3��IΪAl(OH)3��C��D�ڹ��������·�Ӧ����G����ӦΪCl2��H2�ķ�Ӧ��GΪHCl������A���ճ������в���ȱ�ٵ����ʣ�Ҳ�ǻ��������ϵ���Ҫԭ�ϣ�AΪ�Ȼ��ƣ���ⱥ��ʳ��ˮ����H2��Cl2����NaOH����BΪNaOH��CΪH2��DΪCl2��EΪ���NaCl�IJ��ӦΪNa��HΪ������KΪNa2O2������X��������Ӧ����������������XΪFe(OH)2��JΪFeCl2��BΪFe��
(1)Z��L��ӦΪ2Al+Fe2O3![]() 2Fe+Al2O3��Ϊ��Ҫ�����ȷ�Ӧ���ʴ�Ϊ�����ȷ�Ӧ��
2Fe+Al2O3��Ϊ��Ҫ�����ȷ�Ӧ���ʴ�Ϊ�����ȷ�Ӧ��
(2)��������Ϊ���ӻ�����������к��й�����������ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(3)��������ǿ�ᷴӦ��Ҳ����ǿ�Ӧ���ֱ������κ�ˮ��������������Һ��Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
(4)���������������̼��Ӧ����̼���ƺ�����������ʽΪ��2Na2O2+2CO2=2Na2CO3+O2�����ʴ�Ϊ��2Na2O2+2CO2=2Na2CO3+O2��
(5)NaClO�ڼ��������¾���ǿ�������ɽ�һ������2Fe(OH)3����Ӧ�����ӷ���ʽΪ��2Fe(OH)3+3ClO-+4OH-=2FeO42-+3Cl-+5H2O���ʴ�Ϊ��2Fe(OH)3+3ClO-+4OH-=2FeO42-+3Cl-+5H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�