��Ŀ����
����ѭ���ֽ�ˮ������Ҫ�漰���·�Ӧ
I SO2��2H2O��I2=H2SO4��2HI
II 2HI H2��I2
H2��I2
III 2H2SO4=2SO2��O2��2H2O
��1������������Ӧ�������ж���ȷ����__________
a.��ӦIII���ڳ����½��� b.��ӦI��SO2�����Ա�HIǿ
c.ѭ���������貹��H2O d.ѭ�������в���1mol O2��ͬʱ����1 molH2
��2��һ���¶��£���1L�ܱ������м���1 molHI��g����������ӦII��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��
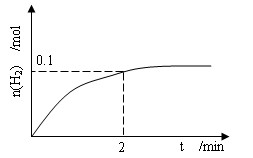
0��2min�Ѽ�������ƽ����Ӧ����v(HI)=_____________�����¶��£�H2(g)��I2(g) 2HI(g)��ƽ�ⳣ��K=__________��
2HI(g)��ƽ�ⳣ��K=__________��
��ͬ�¶��£�����ʼ����2HI(g)�����ʵ�����ԭ����2������_____��ԭ����2����
a.ƽ�ⳣ�� b.HI��ƽ��Ũ�� c.�ﵽƽ���ʱ�� d.ƽ��ʱH2���������
��3��ʵ������Zn��ϡ������H2����Ӧʱ����Һ��ˮ�ĵ���ƽ��________�ƶ�����������ҡ������������������������Լ��е�__________������H2�����ʽ�����
(4)��H2Ϊȼ�Ͽ���������ȼ�ϵ�ء�
��֪ 2H2��g��+O2��g��=2H2O(1) ��H=-572kj��mol-1
ij����ȼ�ϵ���ͷ�228.8kj����ʱ������1molҺ̬ˮ���õ�ص�����ת����Ϊ ��
��
I SO2��2H2O��I2=H2SO4��2HI
II 2HI
 H2��I2
H2��I2III 2H2SO4=2SO2��O2��2H2O
��1������������Ӧ�������ж���ȷ����__________
a.��ӦIII���ڳ����½��� b.��ӦI��SO2�����Ա�HIǿ
c.ѭ���������貹��H2O d.ѭ�������в���1mol O2��ͬʱ����1 molH2
��2��һ���¶��£���1L�ܱ������м���1 molHI��g����������ӦII��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��

0��2min�Ѽ�������ƽ����Ӧ����v(HI)=_____________�����¶��£�H2(g)��I2(g)
 2HI(g)��ƽ�ⳣ��K=__________��
2HI(g)��ƽ�ⳣ��K=__________����ͬ�¶��£�����ʼ����2HI(g)�����ʵ�����ԭ����2������_____��ԭ����2����
a.ƽ�ⳣ�� b.HI��ƽ��Ũ�� c.�ﵽƽ���ʱ�� d.ƽ��ʱH2���������
��3��ʵ������Zn��ϡ������H2����Ӧʱ����Һ��ˮ�ĵ���ƽ��________�ƶ�����������ҡ������������������������Լ��е�__________������H2�����ʽ�����
| A��NaNO3 | B��CuSO4 | C��Na2SO4 | D��NaHSO3 |
��֪ 2H2��g��+O2��g��=2H2O(1) ��H=-572kj��mol-1
ij����ȼ�ϵ���ͷ�228.8kj����ʱ������1molҺ̬ˮ���õ�ص�����ת����Ϊ
 ��
����1��c
��2��0.1mol��L-1��min-1
��3�����ң�b
��4��80%
��

��ϰ��ϵ�д�
 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
�����Ŀ

 ����
����