��Ŀ����
��֪�����淴ӦN����g��+3H2��g��![]() 2NH3��g����H<0�����мס��������ݻ���ͬ�Ҳ��������ܱ���������������м���1mol N2��g����3mol H2��g������һ�������·�����Ӧ���ﵽƽ��ʱ�ų�����ΪQ1kJ������ͬ�����£����������м���2mol NH3��g����������Ӧ���ﵽƽ��ʱ��������ΪQ2kJ����Q1=3Q2��������������ȷ���ǣ� ��
2NH3��g����H<0�����мס��������ݻ���ͬ�Ҳ��������ܱ���������������м���1mol N2��g����3mol H2��g������һ�������·�����Ӧ���ﵽƽ��ʱ�ų�����ΪQ1kJ������ͬ�����£����������м���2mol NH3��g����������Ӧ���ﵽƽ��ʱ��������ΪQ2kJ����Q1=3Q2��������������ȷ���ǣ� ��
A����ƽ��ʱ����N����ת����Ϊ75��
B����ƽ��ʱ�ס�����NH�������������>��
C���ﵽƽ����������м���0.25mol N2��g����0.75mol H2��g����1.5mol NH3��g����ƽ��������N2�ķ����ƶ�
D�����з�Ӧ���Ȼ�ѧ����ʽΪ2NH3��g��![]() N2��g��+3H2��g����H=+Q2kJ/mol
N2��g��+3H2��g����H=+Q2kJ/mol
A
����:

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 qC��g�����ܱ������н��У���ͼ��ʾ��Ӧ�ڲ�ͬʱ��t���¶�T��ѹǿP�뷴Ӧ��B����������Ĺ�ϵ���ߣ�����ͼ����գ���1������2������3�������������������=������
qC��g�����ܱ������н��У���ͼ��ʾ��Ӧ�ڲ�ͬʱ��t���¶�T��ѹǿP�뷴Ӧ��B����������Ĺ�ϵ���ߣ�����ͼ����գ���1������2������3�������������������=������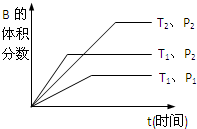
 ��1����4molSO2��2molO2����2L���ܱ������У���һ�������·�����Ӧ����10s��ﵽƽ�⣬���SO3��Ũ��Ϊ0.6mol?L-1����ش��������⣺
��1����4molSO2��2molO2����2L���ܱ������У���һ�������·�����Ӧ����10s��ﵽƽ�⣬���SO3��Ũ��Ϊ0.6mol?L-1����ش��������⣺ ��֪ij���淴Ӧ��mA��g��+nB��g��?pC��g����H ���ܱ��������н��У���ͼ��ʾ����Ӧ�ڲ�ͬʱ��t���¶�T��ѹǿP�뷴Ӧ��B�ڻ�������еİٷֺ�����B%���Ĺ�ϵ���ߣ������߷��������ж���ȷ���ǣ�������
��֪ij���淴Ӧ��mA��g��+nB��g��?pC��g����H ���ܱ��������н��У���ͼ��ʾ����Ӧ�ڲ�ͬʱ��t���¶�T��ѹǿP�뷴Ӧ��B�ڻ�������еİٷֺ�����B%���Ĺ�ϵ���ߣ������߷��������ж���ȷ���ǣ������� ��֪ij���淴Ӧ��mA��g��+nB��g�� xC��g����H=Q kJ/mol�����ܱ������н�����ͼ��
��֪ij���淴Ӧ��mA��g��+nB��g�� xC��g����H=Q kJ/mol�����ܱ������н�����ͼ��