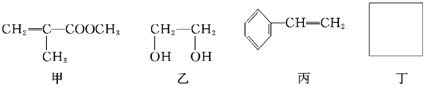
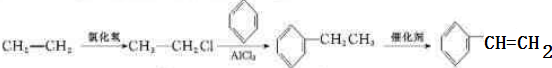
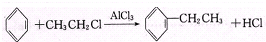
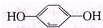
��Ŀ����
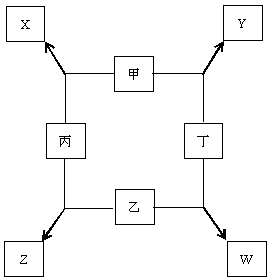
��8�֣��ס��ҡ����������ֵ����ڵ�ȼ������������������X��Y��Z��W���ֻ����ת����ϵ��ͼ��ʾ,��֪��

�ټס��ҡ�����Ϊǰ������Ԫ�صĵ��ʣ������¾�Ϊ��̬��
�����ճ������е�һ�ֳ���������
�ڳ����£�X����ɫҺ�壬Y�Ǻ�ɫ���壻
�۱�������ȼ�շ�����ɫ�Ļ��棬��������ȼ�������ػ�ɫ
���̣�W��ˮ��Һ�ʻ�ɫ��
�ش��������⣺
(1)�� д���������ʵĻ�ѧʽ����X��������������W��������������
��2�����ҵ���ͨ����ɫʯ����Һ�У�������������������������������������
��3��д����������Z��ˮ��Һ��Ӧ�Ļ�ѧ����ʽ����������������������������������������
���𰸡�
H2O FeCl3
��Һ�ȱ�����ɫ
Fe+2HCl= FeCl2+H2
��������

��ϰ��ϵ�д�
�����Ŀ