��Ŀ����
��16�֣�A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������Aԭ�Ӻ��������ӣ�A��E��D��F�ֱ�ͬ���壬��B��D ����������֮��Ϊ2��3���Իش��������⣺
��1��AԪ�ص������� ��EԪ�������ڱ��е�λ���� ��
��2��C��D��F����̬�⻯���ȶ�����ǿ������˳���� ���ѧʽ����
��3��E����������D������ȼ�����ɵĻ�����ĵ���ʽ�� ��
��4��������X��Y����A��D��E��F����Ԫ����ɡ�
��X��Y������ ���������ӡ����ۡ�����
��X��Y��ˮ��Һ���Ϸ�����Ӧ�����ӷ���ʽΪ ��
��5��������E2F��ˮ��Һ�е���˫��ˮ��ϡ���ᣬ���ȣ��е������ɡ������ӷ�Ӧ����ʽΪ�� ��
��6����A2D�����У�ÿ�����������ڵ�4�������γ��������֪�þ���������ȣ�����ֱ�ӱ��ͬ�¶�����ʱ��Ҫ���յ������������þ���������ȣ���51 kJ/mol��������⣬���Ӽ仹���ڷ��»���(11 kJ/mol)����þ���������ġ����ܡ���____kJ/mol��
��1��AԪ�ص������� ��EԪ�������ڱ��е�λ���� ��
��2��C��D��F����̬�⻯���ȶ�����ǿ������˳���� ���ѧʽ����
��3��E����������D������ȼ�����ɵĻ�����ĵ���ʽ�� ��
��4��������X��Y����A��D��E��F����Ԫ����ɡ�
��X��Y������ ���������ӡ����ۡ�����
��X��Y��ˮ��Һ���Ϸ�����Ӧ�����ӷ���ʽΪ ��
��5��������E2F��ˮ��Һ�е���˫��ˮ��ϡ���ᣬ���ȣ��е������ɡ������ӷ�Ӧ����ʽΪ�� ��
��6����A2D�����У�ÿ�����������ڵ�4�������γ��������֪�þ���������ȣ�����ֱ�ӱ��ͬ�¶�����ʱ��Ҫ���յ������������þ���������ȣ���51 kJ/mol��������⣬���Ӽ仹���ڷ��»���(11 kJ/mol)����þ���������ġ����ܡ���____kJ/mol��
��1���� ��1�֣���дH��H2�֣�
�������ڢ�A�壨2�֣����������ڡ���3���ھ��ɣ���A�����֣�
��2��H2O��NH3��H2S��2�֣���������ǿ�����֣�
��3��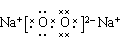 ��2�֣�
��2�֣�
��4�������ӣ�1�֣���H����HSO3�� H2O��SO2����2�֣�
��5��S2����H2O2��2H�� S����2H2O��2�֣������Ȼ���ȷ��ſ�1�֣�
S����2H2O��2�֣������Ȼ���ȷ��ſ�1�֣�
��6��20��2�֣�
�������ڢ�A�壨2�֣����������ڡ���3���ھ��ɣ���A�����֣�
��2��H2O��NH3��H2S��2�֣���������ǿ�����֣�
��3��
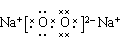 ��2�֣�
��2�֣���4�������ӣ�1�֣���H����HSO3��
��5��S2����H2O2��2H��
 S����2H2O��2�֣������Ȼ���ȷ��ſ�1�֣�
S����2H2O��2�֣������Ȼ���ȷ��ſ�1�֣���6��20��2�֣�
ԭ�Ӻ���������ֻ��H����A����Ԫ�ء�A��E����E��ԭ����������B��C��D�ģ�����Eֻ����Na��B��D ����������֮��Ϊ2��3����ΪD��ԭ������С��Na������Dλ�ڵڶ����ڡ���B��D ��������������2�� 3����C�Ͳ��ܴ��ڣ�����B��D ��������������4��6����B��C��D��O����C��N��F��S��
��1����
��2���ǽ�����Խǿ���⻯����ȶ��Ծ�Խǿ���ǽ�������O��N��S��
��3���Ƶ�ȼ�ղ����ǹ������ƣ��������Ӽ��ͷǼ��Լ���
��4�����ǻ��õĽ�����������H��O��Na��S�γɵĻ�����һ�������ӻ�������Ƿֱ�ΪNaHSO4��NaHSO3��
��5��Na2S��S�Ļ��ϼ۴�����ͼ�̬�����л�ԭ�ԣ���˫��ˮ���������ԣ����߷���������ԭ��Ӧ��
��6��ÿ��ˮ���������ڵ�4�������γ��������ƽ��ÿ��ˮ�����γɵ������2�����������������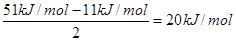 ��
��
��1����
��2���ǽ�����Խǿ���⻯����ȶ��Ծ�Խǿ���ǽ�������O��N��S��
��3���Ƶ�ȼ�ղ����ǹ������ƣ��������Ӽ��ͷǼ��Լ���
��4�����ǻ��õĽ�����������H��O��Na��S�γɵĻ�����һ�������ӻ�������Ƿֱ�ΪNaHSO4��NaHSO3��
��5��Na2S��S�Ļ��ϼ۴�����ͼ�̬�����л�ԭ�ԣ���˫��ˮ���������ԣ����߷���������ԭ��Ӧ��
��6��ÿ��ˮ���������ڵ�4�������γ��������ƽ��ÿ��ˮ�����γɵ������2�����������������
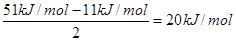 ��
��
��ϰ��ϵ�д�
�����Ŀ
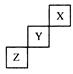
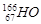 ����Чұ�Ƹΰ�����ͬλ��ԭ�Ӻ��ڵ���������������֮��Ϊ�� ��
����Чұ�Ƹΰ�����ͬλ��ԭ�Ӻ��ڵ���������������֮��Ϊ�� ��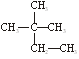 �۰��� ������ ��
�۰��� ������ ��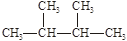
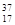 Cl ��
Cl ��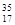 Cl �����
Cl ����� 