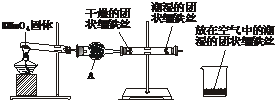
��Ŀ����
�������DZȽϳ���������ijʵ��С��Ϊ�о����������������������¿��١����еķ��������ȼ������װ�õ������ԣ�Ȼ����ͼ���Ӻ�װ�ã���ȼ�ƾ��Ƹ�ҩƷ���ȣ�����3min���ң��۲쵽��ʵ������Ϊ
��ֱ�Թ���������ˮ�����Ĺ�����˿������ɫ��ûҰ���������ʴ
��ֱ�Թ��и������˿������Ȼ������û�з�����ʴ
���ձ��г�ʪ����˿������Ȼ������
������������⣺
��1������������Ӵ��Ľ��ʲ�ͬ��������ʴ�ֳɲ�ͬ���ͣ���ʵ��������������____________���ܱ�ʾ��ԭ���ķ�Ӧ����ʽΪ__________________________________��
��2������A������Ϊ____________������װ��ҩƷ������____________����������____________��
��3���ɴ�ʵ���֪�����������������Ϊ__________________________________�����������������һ����Ҫ������____________��
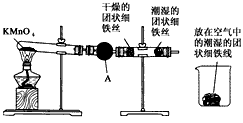
��ֱ�Թ��и������˿������Ȼ������û�з�����ʴ
���ձ��г�ʪ����˿������Ȼ������
������������⣺
��1������������Ӵ��Ľ��ʲ�ͬ��������ʴ�ֳɲ�ͬ���ͣ���ʵ��������������____________���ܱ�ʾ��ԭ���ķ�Ӧ����ʽΪ__________________________________��
��2������A������Ϊ____________������װ��ҩƷ������____________����������____________��
��3���ɴ�ʵ���֪�����������������Ϊ__________________________________�����������������һ����Ҫ������____________��
��1���绯ѧ��ʴ��������2Fe-4e-==2Fe2+��������2H2O+O2+4e-==4OH-
��2�����θ���ܣ���ʯ��(����ˮCaCl2)������O2
��3����O2��H2O�Ӵ�������Ũ��
��2�����θ���ܣ���ʯ��(����ˮCaCl2)������O2
��3����O2��H2O�Ӵ�������Ũ��

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ