��Ŀ����
��ȸʯ��ʯ������Ȼ����ڵ�����̼������ͭ�����ǵĻ�ѧ��ɿɱ�ʾΪxCuCO3?yCu��OH��2��x��yΪ����������x��2��y��2������1����ȸʯ��ʯ��ֱ���������ᷴӦʱ����ȸʯ���õ���������ʵ��������ɵ�CO2�����ʵ���֮��Ϊ4��1��ʯ�����Ϊ3��1�����������ǵĻ�ѧ��ɣ���ȸʯ________��ʯ��_______________��
��2�����п�ȸʯ��ʯ��Ļ����Ʒ��ȡ���ݵ���������Ʒ����һ���м���������ᣬ����CO23.36L����״������������һ����Ʒʹ����ȫ�ֽ⣬�õ�CuO 20g����ͨ������ȷ���û�����п�ȸʯ��ʯ������ʵ���֮�ȡ�
��1�������غ�õ���ϵʽ��CuCO3�D2HCl�DCO2��Cu��OH��2�D2HCl��
���ȸʯ�Ļ�ѧʽΪxCuCO3?yCu��OH��2���Ҽٶ������ʵ���Ϊ1mol����CuCO3��Cu��OH��2�����ʵ����ֱ�Ϊxmol��ymol����������
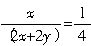 ��������
��������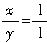 �����ȸʯ�Ļ�ѧʽΪCuCO3?Cu��OH��2��
�����ȸʯ�Ļ�ѧʽΪCuCO3?Cu��OH��2������ʯ��Ļ�ѧʽΪxCuCO3?yCu��OH��2���Ҽٶ������ʵ���Ϊ1mol����CuCO3��Cu��OH��2�����ʵ����ֱ�Ϊxmol��ymol����������
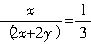 ��������
��������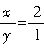 �� ��ʯ��Ļ�ѧʽΪ2CuCO3?Cu��OH��2��
�� ��ʯ��Ļ�ѧʽΪ2CuCO3?Cu��OH��2�� ��2����ÿ����Ʒ��n��CuCO3?Cu��OH��2��=amol��n��2CuCO3?Cu��OH��2��=bmol����ÿ����Ʒ��n��CuCO3��=��a+2b��mol��n��Cu��OH��2��=��a+b��mol��
����̼ԭ���غ�ã���a+2b��mol=3.36L /22.4L?mol-1������ͭԭ���غ�ã���a+2b��mol+��a+b��mol=20g/80
g?mol-1��������ã�a=b=0.05�����ȸʯ��ʯ������ʵ���֮��Ϊ1��1��
�����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ