��Ŀ����
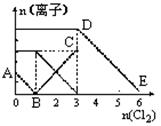
��100 mL FeI2��Һ����ͨ��Cl2������������I2��Fe3����IO3-������Fe3����I2�����ʵ�����n(Cl2)�ı仯��ͼ��ʾ����ش��������⣺

��1����ͼ��֪��I����Fe2����I2�������ӵĻ�ԭ����ǿ������˳��Ϊ________��________��________��
��2����n(Cl2)��0.12 molʱ����Һ�е�������ҪΪ________________________________��
�ӿ�ʼͨ��Cl2��n(Cl2)��0.12 molʱ���ܷ�Ӧ�Ļ�ѧ����ʽΪ______________________��
��3������Һ��n(Cl��)��n(IO3-)��8��1ʱ��ͨ���Cl2�ڱ�״���µ����Ϊ________��

��1����ͼ��֪��I����Fe2����I2�������ӵĻ�ԭ����ǿ������˳��Ϊ________��________��________��
��2����n(Cl2)��0.12 molʱ����Һ�е�������ҪΪ________________________________��
�ӿ�ʼͨ��Cl2��n(Cl2)��0.12 molʱ���ܷ�Ӧ�Ļ�ѧ����ʽΪ______________________��
��3������Һ��n(Cl��)��n(IO3-)��8��1ʱ��ͨ���Cl2�ڱ�״���µ����Ϊ________��
��1��I����Fe2����I2
��2��Fe2����Fe3����Cl����5FeI2��6Cl2=5I2��2FeCl3��3FeCl2
��3��8.96 L
��2��Fe2����Fe3����Cl����5FeI2��6Cl2=5I2��2FeCl3��3FeCl2
��3��8.96 L
��1������ͼ���֪������I����������Ȼ����Fe2�������Ի�ԭ��˳��ΪI����Fe2����I2����2����ͼ���֪n(I2)��0.1 mol������n(FeI2)��0.1 mol��n(I��)��0.2 mol����ͨ��0.12 mol Cl2��0.24 mol Clʱ��I��ȫ����������Fe2����0.04 mol��������������Һ�е�������Ҫ�У�Fe2����Fe3����Cl���������ʵ����ֱ�Ϊ0.06 mol��0.04 mol��0.24 mol��
I2Ϊ0.1 mol��
n(I2)��n(FeCl3)��n(FeCl2)��0.1��0.04��0.06��5��2��3
����ʽΪ5FeI2��6Cl2=5I2��2FeCl3��3FeCl2��
��3��Fe2�� ��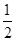 Cl2����I��������
Cl2����I��������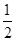 Cl2��I2��2IO3-��5Cl2
Cl2��I2��2IO3-��5Cl2
0.1 mol 0.05 mol 0.2 mol 0.1 mol x 2x 5x
������ã�
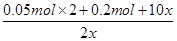 ��8
��8
x��0.05 mol
V(Cl2)��(0.05 mol��0.1 mol��5��0.05 mol)��22.4 L��mol��1��8.96 L��
I2Ϊ0.1 mol��
n(I2)��n(FeCl3)��n(FeCl2)��0.1��0.04��0.06��5��2��3
����ʽΪ5FeI2��6Cl2=5I2��2FeCl3��3FeCl2��
��3��Fe2�� ��
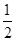 Cl2����I��������
Cl2����I��������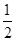 Cl2��I2��2IO3-��5Cl2
Cl2��I2��2IO3-��5Cl20.1 mol 0.05 mol 0.2 mol 0.1 mol x 2x 5x
������ã�
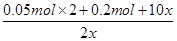 ��8
��8x��0.05 mol
V(Cl2)��(0.05 mol��0.1 mol��5��0.05 mol)��22.4 L��mol��1��8.96 L��

��ϰ��ϵ�д�
�����Ŀ
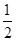
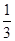
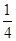
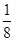
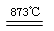 Cl2��2KIO3������˵����ȷ����
Cl2��2KIO3������˵����ȷ����