ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΫΪKClΚΆCuSO4ΝΫ÷÷»ή“ΚΒ»ΧεΜΐΜλΚœΚσΘ§”Ο ·ΡΪΒγΦΪΫχ––ΒγΫβΘ§ΒγΫβΙΐ≥Χ÷–Θ§»ή“ΚpHΥφ ±Φδt±δΜ·ΒΡ«ζœΏ»γΆΦΥυ ΨΘ§‘ρœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
A.’ϊΗωΙΐ≥Χ÷–ΝΫΒγΦΪΖ¥”Π2ClΘ≠Θ≠2eΘ≠=Cl2ΓϋΓΔ2HΘΪΘΪ2eΘ≠=H2Γϋ≤ΜΩ…ΡήΆ§ ±ΖΔ…ζ
B.ΒγΫβ÷ΝcΒψ ±Θ§ΆυΒγΫβ“Κ÷–Φ”»κ ΝΩCuCl2ΙΧΧεΘ§Φ¥Ω… ΙΒγΫβ“ΚΜ÷Η¥÷Ν‘≠ά¥ΒΡ≈®Ε»
C.abΕΈ±μ ΨΒγΫβΙΐ≥Χ÷–HΘΪ±ΜΜΙ‘≠Θ§pH…œ…ΐ
D.‘≠ΜλΚœ»ή“Κ÷–KClΚΆCuSO4ΒΡ≈®Ε»÷°±»«ΓΚΟΈΣ2ΓΟ1
ΓΨ¥πΑΗΓΩA
ΓΨΫβΈωΓΩ
KClΚΆCuSO4ΝΫ÷÷»ή“ΚΒ»ΧεΜΐΜλΚœΚσΘ§”Ο ·ΡΪΒγΦΪΫχ––ΒγΫβΘ§ΒγΫβΖ÷3ΗωΫΉΕΈΘΚ
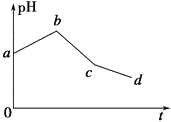
[ΒΎ“ΜΫΉΕΈ]ΒγΫ⬻̷Ά≠Θ§―τΦΪΘΚ¬»άκΉ” ßΒγΉ”Θ§“θΦΪΘΚΆ≠άκΉ”ΒΟΒγΉ”Θ§“ρΈΣΆ≠άκΉ”Υ°Ϋβ Ι»ή“Κœ‘Υα–‘Θ§ΥφΉ≈ΒγΫβΒΡΫχ––Θ§Ά≠άκΉ”ΒΡ≈®Ε»ΫΒΒΆΘ§Υα–‘Φθ»θΘ§pHΫΪ‘ω¥σΘΜ
[ΒΎΕΰΫΉΕΈ]ΒγΫβΝρΥαΆ≠Θ§―τΦΪΘΚ«β―θΗυάκΉ” ßΒγΉ”Θ®ά¥‘¥”ΎΥ°ΒΡΒγάκΘ©Θ§“θΦΪΘΚ»‘»ΜΈΣΆ≠άκΉ”ΒΟΒγΉ”Θ§“ρΈΣ«β―θΗυάκΉ”œϊΚΡΘ§ ΙΥ°»ή“Κ÷–«βάκΉ”≈®Ε»‘ω¥σΘ§pH―ΗΥΌΦθ–ΓΘΜ
[ΒΎ»ΐΫΉΕΈ]ΒγΫβΝρΥαΡΤΘ§―τΦΪΘΚ«β―θΗυάκΉ” ßΒγΉ”Θ§“θΦΪΘΚ«βάκΉ”ΒΟΒγΉ”Θ§ΥϋΟ«ΕΦά¥‘¥”ΎΥ°ΒΡΒγάκΘ§ Β÷ «ΒγΫβΥ°Θ§ΒΦ÷¬»ή“ΚΒΡΧεΜΐΦθ–ΓΘ§ Ι»ή“Κ÷–«βάκΉ”≈®Ε»‘ω¥σΘ§pHΦΧ–χΦθ–Γ
AΘ°ΗυΨί“‘…œΖ÷ΈωΘ§¬»άκΉ”Ζ¥”ΠΆξΘ§Ά≠άκΉ”ΜΙ ΘΘ§Υυ“‘’ϊΗωΙΐ≥Χ÷–ΝΫΒγΦΪΖ¥”ΠΘΚ2Cl--2e-®TCl2ΓϋΘ§2H++2e-®TH2Γϋ≤ΜΩ…ΡήΆ§ ±≥ωœ÷Θ§Ι A’ΐ»ΖΘΜ
BΘ°ΗυΨί“‘…œΖ÷ΈωΘ§ΒγΫβ÷ΝcΒψ ±Θ§‘ΎΒγΫβ“Κ÷–”ΠΦ”»κCuCl2ΙΧΧεΚΆCuOΙΧΧεΘ§Ι B¥μΈσΘΜ
CΘ°ΗυΨί“‘…œΖ÷ΈωΘ§“ρΈΣΆ≠άκΉ”Υ°Ϋβ Ι»ή“Κœ‘Υα–‘Θ§ΥφΉ≈ΒγΫβΒΡΫχ––Θ§Ά≠άκΉ”ΒΡ≈®Ε»ΫΒΒΆΘ§Υα–‘Φθ»θΘ§pHΫΪ‘ω¥σΘ§Ι C¥μΈσΘΜ
DΘ°“ρΈΣ”…ΒΎΕΰΫΉΕΈ―τΦΪ«β―θΗυάκΉ” ßΒγΉ”ΒΡΆ§ ±Θ§“θΦΪ»‘»ΜΈΣΆ≠άκΉ”ΒΟΒγΉ”Θ§Υυ“‘‘≠ΜλΚœ»ή“Κ÷–KClΚΆCuSO4ΒΡ≈®Ε»÷°±»≤Μ «2ΘΚ1Θ§Ι D¥μΈσΘΜ
Ι ―ΓAΓΘ

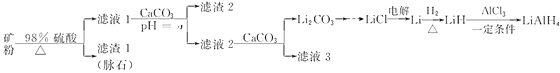
 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΥΡ«β¬Νο°(LiAlH4)≥ΘΉς”–ΜζΚœ≥…ΒΡ÷Ί“ΣΜΙ‘≠ΦΝΓΘ“‘Μ‘ο°Ωσ(÷ς“Σ≥…Ζ÷ «Li2OΓΛAl2O3ΓΛ4SiO2Θ§Κ§…ΌΝΩFe2O3)ΈΣ‘≠ΝœΚœ≥…ΥΡ«β¬Νο°ΒΡΝς≥Χ»γœ¬ΘΚ
“―÷ΣΘΚΔΌΦΗ÷÷Ϋπ τ«β―θΜ·Έο≥ΝΒμΒΡpH»γœ¬±μΥυ ΨΘΚ
Έο÷ | Fe(OH)3 | Al(OH)3 |
ΩΣ Φ≥ΝΒμΒΡpH | 2.3 | 4.0 |
Άξ»Ϊ≥ΝΒμΒΡpH | 3.7 | 6.5 |
ΔΎ≥ΘΈ¬œ¬Θ§Ksp(Li2CO3)ΘΫ2.0ΓΝ10-3Θ§Li2CO3‘ΎΥ°÷–»ήΫβΕ»ΥφΉ≈Έ¬Ε»…ΐΗΏΕχΦθ–ΓΓΘ
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)…œ ωΝς≥Χ÷–Θ§ΧαΗΏΓΑΥαΫΰΓ±ΥΌ¬ ΒΡ¥κ ©”–______________________________________ΓΔ______________________________________________________________________________(–¥ΝΫΧθ)ΘΜΦ”»κCaCO3ΒΡΉς”Ο «__________________Θ§ΓΑaΓ±ΒΡΉν–Γ÷ΒΈΣ___________ΓΘ
(2)…ηΦΤΦρΒΞΖΫΑΗ”…Li2CO3÷Τ±ΗLiClΘΚ__________ΓΘ
(3)–¥≥ωLiHΚΆAlCl3Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______________________(ΧθΦΰ≤ΜΉς“Σ«σ)ΓΘ
(4)”Ο»»Υ°œ¥Β”Li2CO3ΙΧΧεΘ§Εχ≤Μ”ΟάδΥ°œ¥Β”Θ§ΤδΡΩΒΡ «________________ΘΜΦλ―ιΧΦΥαο° «Ζώœ¥ΨΜΒΡ Β―ιΖΫΖ® «_____________________ΓΘ
(5)‘Ύ”–ΜζΚœ≥…÷–Θ§ΜΙ‘≠ΦΝΒΡΜΙ‘≠ΡήΝΠΆ®≥Θ”ΟΓΑ”––ß«βΓ±±μ ΨΘ§ΤδΚ§“ε «1ΩΥΜΙ‘≠ΦΝœύΒ±”ΎΕύ…ΌΩΥ«βΤχΒΡΜΙ‘≠ΡήΝΠΓΘLiAlH4ΒΡΓΑ”––ß«βΓ±ΈΣ_________________(ΫαΙϊ±ΘΝτ2ΈΜ–Γ ΐ)ΓΘ