��Ŀ����
25��ʱ������������Һ��pH���±���ʾ��| ���� | CH3COOH | H2SO3 | HCN |
| ���ʵ���Ũ��mol/L | 0.01 | 0.01 | 0.01 |
| pH | 3.4 | 2.3 | 5.6 |
��1��25��ʱ��Na2SO3��Һ��pH______�������������������=����7����ԭ����______�������ӷ���ʽ��ʾ����
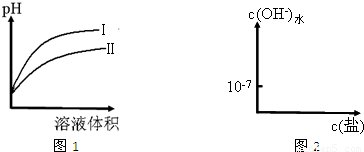
��2��ͼ1�dz����£���ͬpH��CH3COOH��HCN��Һ�ֱ��ˮϡ��ʱ��Һ��pH����Һ����仯�����ߣ�
��ͼ1������______�������ʾ��ˮϡ��HCN��ҺʱpH�ı仯��
�����в�����ʹCH3COOH�ĵ���̶���
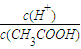 ͬʱ�������______��
ͬʱ�������______��A����ˮϡ����Һ B��������Һ�¶� C����������NaHSO4���� D����������CH3COONa����
����ͼ2�зֱ�������CH3COONa��Һ��NaCN��Һ����ˮ�������c��OH-��������Ũ�ȵı仯���ߣ�
��3�������£����ʵ���Ũ����ͬ��CH3COONa��Һ��NaCN��Һ�У�CH3COONa��Һ�и�����Ũ��֮��______�����������������=����NaCN��Һ�и�����Ũ��֮�ͣ�
��4����֪��HCN��aq��
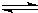 H+��aq��+CN-��aq����H=+43.5kJ?mol-1
H+��aq��+CN-��aq����H=+43.5kJ?mol-1H+��aq��+OH-��aq��=H2O��1����H=-57.3kJ?mol-1��NaCN����Һ�з���ˮ����Ȼ�ѧ����ʽ��
CN-��aq��+H2O��1��
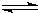 HCN��aq��+OH-��aq���ġ�H=______���÷�Ӧ25��ʱ��ƽ�ⳣ��K=______��
HCN��aq��+OH-��aq���ġ�H=______���÷�Ӧ25��ʱ��ƽ�ⳣ��K=______�������������ֵ����֪10-5.6=2.5×10-6��
��2�����ɱ����е����ݷ������Ե�ǿ�������ˮ�ٽ�������ʵĵ��룬���Խ�ǿ����Ũ�ȱ仯��
�ڸ���Ӱ��������ƽ���ƶ���������������
�۸�������ˮ��Ĺ��ɿ�֪����Խ����ˮ��̶�Խ���ε�Ũ�ȴ�ˮ�����ɵ����������ӵ�Ũ�ȴ�
��3��ˮ��̶ȴ����Һ�е�������Ũ��С��Ȼ����ݵ���غ㼰���ӵ����ʵ���Ũ����������
��4�����ø�˹���������㷴Ӧ�ȣ������û�ѧ��Ӧ��ƽ�ⳣ������볣����Kw�Ĺ�ϵ������ˮ��ƽ�ⳣ����
����⣺��1��25��ʱ��0.01mol/L�������pH=2.3����������Ϊ���ᣬ����������Ϊǿ�������Σ���SO32-+H2O
 HSO3-+OH-��֪��Һ�Լ��ԣ�pH��7��
HSO3-+OH-��֪��Һ�Լ��ԣ�pH��7���ʴ�Ϊ������SO32-+H2O
 HSO3-+OH-��
HSO3-+OH-����2�����ɱ����е����ݿ�֪����ͬŨ�ȵ�CH3COOH��HCN��Һ��HCN��pH����HCN�����Ը�������ͬpH��CH3COOH��HCN��Һ�ֱ��ˮϡ��ʱ�������������
Ũ�ȱ仯����HCN�������ӵ�Ũ�ȱ仯��������pH�仯���������ߢ��ʾ��ˮϡ��HCN��ҺʱpH�ı仯���ʴ�Ϊ����
����CH3COOH
 H++CH3COO-����ˮϡ�ͣ��ٽ����룬����ƽ�������ƶ�������̶�����
H++CH3COO-����ˮϡ�ͣ��ٽ����룬����ƽ�������ƶ�������̶�����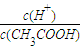 ����A��ȷ��
����A��ȷ����������ʵĵ��������ȵģ��������¶ȣ�����ƽ�������ƶ�������̶�����
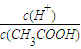 ����B��ȷ��
����B��ȷ����������NaHSO4���壬H+��Ũ��������ƽ�������ƶ�������̶ȼ�С����C����
��������CH3COONa���壬CH3COO-��Ũ��������ƽ�������ƶ�������̶ȼ�С����C����
�ʴ�Ϊ��AB��
���ɱ����е����ݿ�֪������CH3COOH��HCN��CH3COONa��Һ��NaCN��Һ�ж�ˮ������OH-������ͬŨ�ȵ���ʱ��NaCN��Һˮ�����ɵ�OH-����Ũ�ȴ�
���ε�Ũ��Խ��ˮ�����ɵ�OH-����Ũ��Ҳ��Խ�ʴ�Ϊ��
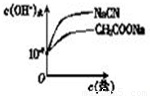 ��
����3�����ʵ���Ũ����ͬ��CH3COONa��Һ��NaCN��Һ�У��ɵ���غ��֪CH3COONa��Һ�д���c��Na+��+c��H+��=c��CH3COO-��+c��OH-����
NaCN��Һ�д���c��Na+��+c��H+��=c��CN-��+c��OH-����������Һ�е�����Ũ��֮�͵ıȽϼ�Ϊ2c��Na+��+2c��H+���ıȽϣ�
�����ʵ���Ũ��ʱ��NaCN��Һˮ�����ɵ�OH-����Ũ�ȴ���2c��H+����С������CH3COONa��Һ�и�����Ũ��֮�ʹʴ�Ϊ������
��4��HCN��aq��
 H+��aq��+CN-��aq����H=+43.5kJ?mol-1�٣�
H+��aq��+CN-��aq����H=+43.5kJ?mol-1�٣�H+��aq��+OH-��aq��=H2O��1����H=-57.3kJ?mol-1�ڣ�
�ɸ�˹���ɿ�֪��ӦCN-��aq��+H2O��1��
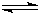 HCN��aq��+OH-��aq������-��+��-�ڣ��õ�����Ӧ��
HCN��aq��+OH-��aq������-��+��-�ڣ��õ�����Ӧ����H�T-��+43.5kJ?mol-1��+57.3kJ?mol-1=+13.8 kJ/mol��K=
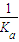 ×Kw=
×Kw=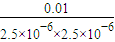 ×10-14=1.6×10-5��
×10-14=1.6×10-5���ʴ�Ϊ��+13.8 kJ/mol��1.6×10-5��
������������ѣ������֪ʶ��϶࣬��������ˮ���л��Ľ����һ�𣬲����÷�Ӧ�Ĺ�ϵ���㷴Ӧ�Ⱥ�ˮ��ƽ�ⳣ�����Ϻõ�ѵ��ѧ�����������㡢ͼ�������ۺ�������

ij���Ṥ�������Է�ˮ���飨As��Ԫ�غ������ߣ�Ϊ��������ŷţ����û�ѧ���������������ˮ�������������£�
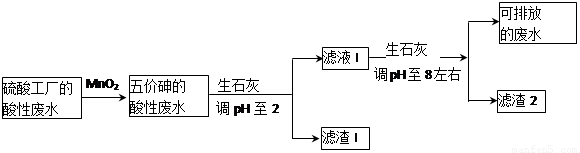
����������±�����ش��������⣺
��1�������ε�Ksp
|
������ |
Ksp |
|
Ca3(AsO4)2 |
6.8��10��19 |
|
CaSO4 |
9.1��10��6 |
|
FeAsO4 |
5.7��10��21 |
��2��������Ⱦ���ŷ�Ũ�ȼ������ŷű�
|
��Ⱦ�� |
H2SO4 |
As |
|
��ˮŨ�� |
29.4g/L |
1.6g��L��1 |
|
�ŷű� |
pH 6��9 |
0.5mg��L��1 |
��1�������Ṥ���ŷŵķ�ˮ����������ʵ���Ũ��c(H2SO4)= mol��L��1��
��2�������Է�ˮ��Fe3+��Ũ��Ϊ1.0��10��4mol��L��1��c(AsO43��)= mol��L��1��
��3�������ŷų������Է�ˮ�е������飨H3AsO3���ᣩ���׳�������Ͷ��MnO2�Ƚ�������������飨H3AsO4���ᣩ��MnO2����ԭΪMn2������Ӧ�����ӷ���ʽΪ ��
��4���ڴ��������ˮʱ���÷ֶ�ʽ�������ˮ��Ͷ����ʯ�ҵ���pH��2����Ͷ����ʯ�ҽ�pH���ڵ�8����ʹ�������Ca3(AsO4)2��ʽ������
�ٽ�pH���ڵ�2ʱ��ˮ���д�������������������Ҫ�ɷֵĻ�ѧʽΪ ��
��Ca3(AsO4)2��pH���ڵ�8���Ҳſ�ʼ������ԭ��Ϊ
��
�����ᣨH3AsO4���ֲ������ƽ�ⳣ��(25��)Ϊ��K1=5.6��10��3 K2=1.7��10��7 K3=4.0��10��12�������������ƽ�ⳣ���ı���ʽΪK3=
��Na3AsO4�ĵ�һ��ˮ������ӷ���ʽΪ��AsO43��+H2O HAsO42��+OH�����ò�ˮ���ƽ�ⳣ����25�棩Ϊ��
��������λ��Ч���֣���
HAsO42��+OH�����ò�ˮ���ƽ�ⳣ����25�棩Ϊ��
��������λ��Ч���֣���