��Ŀ����
13����1��һ���¶��£���2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��仯��������ͼ��ʾ��
�ٷ�Ӧ��ʼ��ƽ�⣬��Z��ʾ�ķ�Ӧ����Ϊ0.079mol•L-1•s-1��
��Y��ת����Ϊ79%��
�۸÷�Ӧ�Ļ�ѧ����ʽΪX+Y?2Z��
��2������ͭ����пƬ��200mLϡ�������ԭ��أ�����ѧ��ת��Ϊ���ܣ���ͭ���Ϲ�����3.36L����״��������ʱ������ǡ��ȫ�������ģ���ش��������⣺
�ٸ�ԭ��ص�������ӦʽΪ��2H++2e-��H2����
�ڼ���ԭϡ��������ʵ���Ũ��Ϊ0.75mol/L��
������ͭ��������Ƭ���������ʽ����������ܷ����ԭ����أ��ܣ���ܡ����ܡ��� �������ԭ��أ���ԭ��صĸ�����Ӧʽ��Zn-2e-=Zn2+��
���� ��1����ͼ����Կ�������Ӧ��x��Y�����ʵ������٣�ӦΪ��Ӧ�z�����ʵ������࣬ӦΪ���������Y��X��Z=��1.20mol-0.41mol������1.0mol-0.21mol����1.58mol=1��1��2����Ӧ�Ļ�ѧ����ʽΪX+Y?2Z���Դ˽����⣻
��2����ͭ����пƬ��200mlϡ�������ԭ��أ�����п�Ǹ�����ͭ�������������Ϸ�����ԭ��Ӧ��
�ڸ��������缫�ϵķŵ�������������㣻
�۽�ͭ��������Ƭ������пƬ��200mlϡ�������ԭ��أ�����п�Ǹ��������������������Ϸ���������Ӧ���ݴ˻ش�
��� �⣺��1���ٷ�Ӧ��ʼ��10s����Z��ʾ�ķ�Ӧ����Ϊ��v=$\frac{\frac{1.58mol}{2L}}{10s}$=0.079mol/��L•s����
�ʴ�Ϊ��0.079mol/��L•s����
�ڷ�Ӧ��ʼ��10sʱ��Y��ת����Ϊ$\frac{1.0mol-0.21mol}{1.0mol}$��100%=79%��
�ʴ�Ϊ��79%��
����ͼ����Կ���X��Y�����ʵ�����С��Z�����ʵ������࣬��X��YΪ��Ӧ�ZΪ�����
��ѧ��Ӧ�и����ʵ����ʵ����仯���뻯ѧ������֮�ȳ����ȣ�
����Y��X��Z=��1.20mol-0.41mol������1.0mol-0.21mol����1.58mol=1��1��2����Ӧ�Ļ�ѧ����ʽΪX+Y?2Z��
�ʴ�Ϊ��X+Y?2Z��
��2����ͭ����пƬ��200mlϡ�������ԭ��أ�����п�Ǹ�����ͭ��������ԭ��ص������缫�����ĵ缫��Ӧ�ǣ�������2H++2e-=H2����������Zn=Zn2++2e-��
�ʴ�Ϊ��2H++2e-��H2����
��ͭƬ����������3.36L����״������������0.15mol��������2H++2e-��H2��������ת�Ƶĵ���Ϊ0.3mol����Һ�е���������0.3mol������ԭϡ������Һ��H+�����ʵ���Ũ��=$\frac{0.3mol}{0.2L}$=1.5mol/L��ԭϡ��������ʵ���Ũ��Ϊ0.75mol/L��
�ʴ�Ϊ��0.75mol/L��
������ͭ��������Ƭ������пƬ��200mlϡ�������ԭ��أ�����п�Ǹ��������������������Ϸ���������Ӧ���缫��ӦʽΪ��Zn-2e-=Zn2+��
�ʴ�Ϊ���ܣ�Zn-2e-=Zn2+��
���� ���⿼�����ʵ�����ʱ��ı仯���ߡ��Լ���ѧƽ��ļ��㡢ԭ��صĹ���ԭ���Լ�����Ũ�ȵļ��㷽���ǽ���Ĺؼ���������ѧ���ķ��������ͼ��������Ŀ��飬��Ŀ�Ѷ��еȣ�ע����ջ�ѧ����ʽ���жϷ�����

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�| A�� | ���Ϊ��صĸ�����ʯī����ص����� | |
| B�� | ��ع��������У�SOCl2����ԭΪLi2SO3 | |
| C�� | �õ���ڻ���Ӧ������ˮ����������Ӱ���ص����� | |
| D�� | ��ع���ʱ����ṩ�ĵ��ӵ����ʵ���������������ʵ���֮����4��1 |

| A�� | ��Ӧ�٢��ж����ڣ���S��0 | B�� | ��Ӧ�ڸ���ʱ�����Է����� | ||
| C�� | ��ͨ������£��٢ڶ����Է����� | D�� | ��Ӧ��һ�������Է����� |
| A�� | ����Ũ����������ĵ����� | |
| B�� | ����ˮ������ά��ˮ��õ������ղ�����ͬ | |
| C�� | �����ʡ���ά�ء����ǡ���֬���Ǹ߷��ӻ����� | |
| D�� | ��ҵ��������֬�ڼ�Ĵ�������ˮ���������� |
��CH3CH=CH2+Br2$\stackrel{CCl_{4}}{��}$CH3CHBrCH2Br
��CH3CH2OH $��_{��}^{ŨH_{2}SO_{4}}$CH2=CH2+H2O
��

��CH3Cl+Cl2$\stackrel{��}{��}$CH2Cl2+HCl��
| A�� | �٢� | B�� | �ۢ� | C�� | �٢� | D�� | �ڢ� |
| A�� | ���ʯ��ʯī����ͬλ�� | B�� | ������춡�黥��ͬϵ�� | ||
| C�� | ҽ�������ƾ����Ҵ���Ũ��Ϊ95% | D�� | 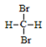 �� ��  ����ͬ���칹�� ����ͬ���칹�� |
| A�� | �÷�Ӧ����ʽ�У�x=1 | B�� | 2minʱ��A��ת����Ϊ50% | ||
| C�� | 2minʱ��A�����ʵ���Ϊ0.75mol | D�� | ��Ӧ����v��B��=0.25 mol•L-1•min-1 |
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��Na2CO3��NaHCO3�Ĺ�������������� | ��ȥNaHCO3�е�Na2CO3 |
| B | ����ʱ��COͨ��װ��SiO2��Ӳ�ʲ����� | ��ȡ�ֹ� |
| C | �����Ƶõ�K2S��Һ�еμ����Ƶ���ˮ | �Ƚ�������ĵõ������� |
| D | �ֱ���2֧ʢ����ˮ���Թ��м�����ͬ��С��þ�������� | �Ƚ�þ���Ľ�����ǿ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ��Դ����������������ٵ��ش���⣮�״���δ����Ҫ����ɫ��Դ֮һ��
��Դ����������������ٵ��ش���⣮�״���δ����Ҫ����ɫ��Դ֮һ��