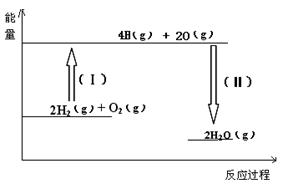
��Ŀ����
������KMnO4��H2C2O4�����ᣩ��Ӧ�о�Ӱ�췴Ӧ���ʵ����أ����ӷ���ʽΪ��2MnO4����5H2C2O4��6H����2Mn2����10CO2����8H2O��һʵ��С����ͨ���ⶨ��λʱ��������CO2�����ʣ�̽��ij��Ӱ�컯ѧ��Ӧ���ʵ����أ����ʵ�鷽�����£�KMnO4��Һ���ữ����
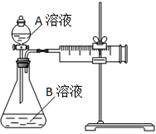
��1����ʵ��̽������ ���ضԻ�ѧ��Ӧ���ʵ�Ӱ�졣��ͬʱ������Ͳ������CO2�������С��ϵ�� �� ����ʵ����ţ���
��2����ʵ�����2 minĩ�ռ���4.48 mL CO2����״���£�������2 minĩ��c(MnO4��)��_______mol��L��1������������Һ���Ϊ50 mL��
��3����ͨ���ⶨһ��ʱ����CO2��������ȽϷ�Ӧ���ʣ���ʵ�黹��ͨ���ⶨ ���Ƚϻ�ѧ��Ӧ���ʡ�
��4��С��ͬѧ���ַ�Ӧ������������ͼ������t1��t2ʱ�������ʱ�����Ҫԭ������ǣ��ٲ���Mn2���Ƿ�Ӧ�Ĵ������� ��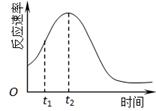

| ʵ����� | A��Һ | B��Һ |
| �� | 20 mL 0.1 mol��L��1H2C2O4��Һ | 30 mL 0.01 mol��L��1KMnO4��Һ |
| �� | 20 mL 0.2 mol��L��1H2C2O4��Һ | 30 mL 0.01 mol��L��1KMnO4��Һ |
��2����ʵ�����2 minĩ�ռ���4.48 mL CO2����״���£�������2 minĩ��c(MnO4��)��_______mol��L��1������������Һ���Ϊ50 mL��
��3����ͨ���ⶨһ��ʱ����CO2��������ȽϷ�Ӧ���ʣ���ʵ�黹��ͨ���ⶨ ���Ƚϻ�ѧ��Ӧ���ʡ�
��4��С��ͬѧ���ַ�Ӧ������������ͼ������t1��t2ʱ�������ʱ�����Ҫԭ������ǣ��ٲ���Mn2���Ƿ�Ӧ�Ĵ������� ��

��10�֣�
��1��Ũ�ȣ�2�֣� �ڣ��٣�2�֣�
��2��0.0092��2�֣�
��3��KMnO4��Һ��ȫ��ɫ����ʱ��������ͬ������������ʱ�䣨2�֣�
��4���÷�Ӧ���ȣ�2�֣�
��1��Ũ�ȣ�2�֣� �ڣ��٣�2�֣�
��2��0.0092��2�֣�
��3��KMnO4��Һ��ȫ��ɫ����ʱ��������ͬ������������ʱ�䣨2�֣�
��4���÷�Ӧ���ȣ�2�֣�
�����������1�����ݱ������������ݷ�����ʵ��̽������Ũ�����ضԻ�ѧ��Ӧ���ʵ�Ӱ�졣Ũ��Խ��Ӧ����Խ�죬���ɵ�CO2�����Խ���ڣ��١�
��2��4.48 mL CO2����0.0002mol�����ݻ�ѧ����ʽ���㣬��Ӧ��MnO4����0.00004mol��ʣ���MnO4����0.0005mol-0.00004mol=0.00046mol����c(MnO4��)��0.0092mol��L��1��
��3��KMnO4������ɫ�ģ��ʻ���ͨ���ⶨKMnO4��Һ��ȫ��ɫ����ʱ��������ͬ������������ʱ�����Ƚϻ�ѧ��Ӧ���ʡ�
��4�������¶Ȼ�ӿ췴Ӧ�����ʡ�
���������⿼����Ӱ�컯ѧ��Ӧ���ʵ����ؼ����㣬�Ѷ��еȣ�ע�������Ӧ���ʵ������ʱ��������ʣ��������ֻ��Ӱ�����ء�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 Si (s)+ 4HCl(g) ��236kJ
Si (s)+ 4HCl(g) ��236kJ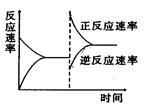
 N2��g��+CO2��g�� ��ij�о�С����ij�ܱյ������������������������䣬��������������Բ��ƣ��м���NO�������Ļ���̿�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����ұ���
N2��g��+CO2��g�� ��ij�о�С����ij�ܱյ������������������������䣬��������������Բ��ƣ��м���NO�������Ļ���̿�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����ұ���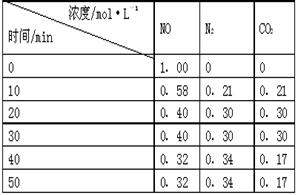
 ?2X Y2(g)����H��0�ﵽ��ƽ�⣬��ֻ�ı�ijһ�����ٴﵽ��ƽ�⣬��Դ˹��������ı�ķ�����ȷ����
?2X Y2(g)����H��0�ﵽ��ƽ�⣬��ֻ�ı�ijһ�����ٴﵽ��ƽ�⣬��Դ˹��������ı�ķ�����ȷ����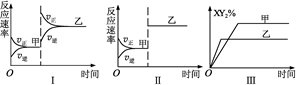
 2NO2 (g) ��H<0��
2NO2 (g) ��H<0��
 PCl3(g)��Cl2(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±�������˵����ȷ����
PCl3(g)��Cl2(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±�������˵����ȷ���� 2C(g)+xD(g)����5min�ﵽƽ�⣬��ʱ����2 mol C�����D��ƽ����Ӧ����Ϊ0.15 mol/(L��min)����ƽ��ʱA�����ʵ���Ũ����____________��B��ת������__________��x��ֵ��___________��
2C(g)+xD(g)����5min�ﵽƽ�⣬��ʱ����2 mol C�����D��ƽ����Ӧ����Ϊ0.15 mol/(L��min)����ƽ��ʱA�����ʵ���Ũ����____________��B��ת������__________��x��ֵ��___________��