��Ŀ����
A��B�������ʻ����ܽ�Ĺ�ϵ��ͼ1��ʾ���������ʾ��ϵ������ɣ�������Ϊ�¶ȣ��������ϣ��¶������ߡ�T1ʱa��B��A�еı�����Һ����ɣ�b��A��B�еı�����Һ����ɣ�T2ʱ��ӦΪc��d����T3Ϊ�ٽ��¶ȣ���ʱA��B��ȫ���ܡ�ͼ��������Ϊ���࣬������Ϊһ�ࣨ������Һ����ijЩ������H2O��(C2H5)3N��C3H5(OH)3�ͼ�-CH3C6H4NH2�е͵��ٽ��¶ȣ���ʾ��ͼ2���������������ʵ���ش��������⣺



 T3
T3

 T2 c d
T2 c d
T1 a b






 18.5�� C
18.5�� C
A 100% 80% 60% 40% 20% 0% H2O (C2H5)3N
0% 20% 40% 60% 80% 100% B
ͼ 1 ͼ 2
2-1 ����ͼ1��T3���ڵ�ԭ��
2-2 ˵��ͼ2��ʾ���������ʴ��ڵ͵��ٽ��¶ȵ�ԭ��
2-3 ����ͼ2��ʾ���������ʵĻ��ܹ��̡�



 T3
T3
 T2 c d
T2 c dT1 a b






 18.5�� C
18.5�� CA 100% 80% 60% 40% 20% 0% H2O (C2H5)3N
0% 20% 40% 60% 80% 100% B
ͼ 1 ͼ 2
2-1 ����ͼ1��T3���ڵ�ԭ��
2-2 ˵��ͼ2��ʾ���������ʴ��ڵ͵��ٽ��¶ȵ�ԭ��
2-3 ����ͼ2��ʾ���������ʵĻ��ܹ��̡�
2-1���ʵ��ܽ��ͨ�������¶ȵ����߶�����������һ�����¶��£�A��B���������ܴﵽ��ȫ���ܡ� ��2�֣�
2-2 ijЩ������H2O��(C2H5)3N��C3H5(OH)3�ͼ�-CH3C6H4NH2���ڵ͵��ٽ��¶ȵ�ԭ���������ڽϵ��¶����γɷ��Ӽ������Ȼ���������¶ȵ����ߣ����Ӽ�������ѣ������ܽ�ȷ�����С�� (4��)
2-3
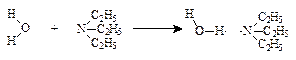 (2��)
(2��)
(����Ϊ1��H2O��2��(C2H5)3N�����γ����Ҳ��2��)
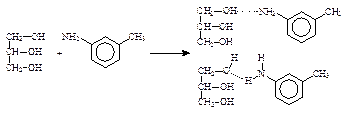 (2��)
(2��)
(���λ�ò�ͬ������1��2��3����������������𰸾�����)
2-2 ijЩ������H2O��(C2H5)3N��C3H5(OH)3�ͼ�-CH3C6H4NH2���ڵ͵��ٽ��¶ȵ�ԭ���������ڽϵ��¶����γɷ��Ӽ������Ȼ���������¶ȵ����ߣ����Ӽ�������ѣ������ܽ�ȷ�����С�� (4��)
2-3
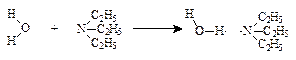 (2��)
(2��)(����Ϊ1��H2O��2��(C2H5)3N�����γ����Ҳ��2��)
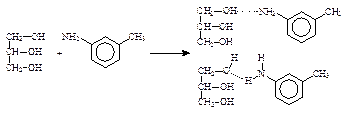 (2��)
(2��)(���λ�ò�ͬ������1��2��3����������������𰸾�����)
�������Ϣ��ͼ�Σ��ɳ�Ϊͼ���⡣��������߶���ͼ���ݵ���Ϣ����ӹ�������������������Ӧץס��������⡪�������ܼ��Ļ����ԣ�Ȼ��Ҫ������ѧ��ѧ�й����ܽ��Ե�һ�������⡪��Ϊʲô�е��ܼ��ܹ����ܣ��е�ȴ���ܣ����Ҫ������ѧ��ѧ���й��¶ȶ��ܽ��Ӱ���һ��ԭ�������ߵ���˼����˳��˼·�ͻ��ҡ���ѧ��ѧ�С����������ܡ���˵���������Ӽ������������������СҲ������ܼ�����ܽ⡣��������ʹ�С���Ҳ�и�˳��������������ٿ��Ǵ�С�Ƿ���������ʲ�����Ͳ����ٿ��Ǵ�С�Ƿ������ͼ1��2�������ܽ������¶ȹ�ϵ�෴����Ϣ��Ӧ��������߷��Ӽ������ʲ�ͬ���������ͼ2�ܼ���ʵ������������������ͼ1���ܼ��������������ʣ�������ͼ1��ͨ����ͼ2����������˲����������ȷ���ͼ2��������ʲô����ķ��Ӽ������������Ǿ��ҵ��˽���Ĺؼ����������

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ
 O2(g)��H2O(l) ��H 3����285.8kJ/mol
O2(g)��H2O(l) ��H 3����285.8kJ/mol