��Ŀ����
Ϊ��̽�����ƾ��壨Na2S?nH2O���ڿ����еı仯��ij��ȤС���ʵ�鲽�輰�����¼���£��ٽ��������ƾ������ڱ������ϣ���¶�ڿ����У����������Ʒ����ʢˮ���ձ��У���������ɫ������Һ����ȡ��������Һ������������У�������ų�������Һ���ֳ��壮
��1���������������¼���Էų�������в��룺
����٣�������H2S������������ڣ�������SO2������������ۿ�����CO2��
����ܣ�������______��������ݣ�������______��
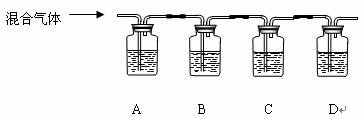
��2�����ų�����������������Ļ���������У�Ũ��ϡ2��Ʒ����Һ��Ũ��ϡ2����ˮ������ʯ��ˮ��Ũ��ϡ2��������Һ���Թ����巢��װ�ã�ϴ��ƿ���Թ�ϴ��װ�ã������һ�����ʵ��������������ɣ����±��м�Ҫд��ʵ�鷽��������ͽ��ۣ�
| ʵ�鷽�� | ���ܵ��������Ӧ�Ľ��� |
| ______ | ______ |
| ______ |
�⣺��1�������Ʊ��ʺ�������ΪNa2SO3��Na2SO4��Na2CO3��Na2S��Na2SO3��Na2SO4��Na2CO3֮�䲻��Ӧ��Na2S��Na2SO3��Na2CO3�����ᷴӦ�ֱ�õ�H2S��SO2��CO2��Na2SO4�������Ӧ����������������ǵ�һ���壬Ҳ�����ǻ�����壬��������岻��ΪSO2��H2S����Ϊ���߷�Ӧ��������������Ի���������ΪH2S��CO2�Ļ������Ҳ����ΪSO2��CO2�Ļ�����壬�ʴ�Ϊ��H2S��CO2�Ļ�����壻SO2 ��CO2�Ļ�����壻
��2����ͨ��Ũ��ˮһ����ɹ۲�H2S����������S����������һ����ɾ�����������SO2���Է�SO2�����һ������ʯ��ˮ�ܽ�CaCO3�����Ź۲죻��ʯ��ˮǰ���װϡ��ˮϴ��װ����Ϊ�˱��ڹ۲�SO2�Ƿ�����һ��������ȫ��ѡϡƷ����Һ�۲���ɫ���ʴ�Ϊ��
��3������������ӵļ��鷽�����μ�BaCl2��Һ�����г�������˵������������ӣ��ʴ�Ϊ��ȡ����ʵ��ۺ�������Һ����BaCl2��Һ�����г�������˵��������Na2SO4������������˵��û������Na2SO4��
��������1���������Ʊ��ʺ�������ΪNa2SO3��Na2SO4��Na2CO3��Na2S��Na2SO3��Na2SO4��Na2CO3֮�䲻��Ӧ��Na2S��Na2SO3��Na2CO3�����ᷴӦ�ֱ�õ�H2S��SO2��CO2��Na2SO4�������Ӧ����������������ǵ�һ���壬Ҳ�����ǻ�����壬��������岻��ΪSO2��H2S����Ϊ���߷�Ӧ������������ݴ˻ش����⣻
��2����ͨ��Ũ��ˮһ����ɹ۲�H2S����������S����������һ����ɾ�����������SO2���Է�SO2�����һ������ʯ��ˮ�ܽ�CaCO3�����Ź۲죻��ʯ��ˮǰ���װϡ��ˮϴ��װ����Ϊ�˱��ڹ۲�SO2�Ƿ�����һ��������ȫ��ѡϡƷ����Һ�۲���ɫ����
��3��������������ӵļ��鷽�����μ�BaCl2��Һ�����г�������˵������������ӣ�
������������Ҫ��������Ļ���������ʣ��ڼ�������ʱҪע��˳��ͬʱҪ��ȥ���ʣ�
��2����ͨ��Ũ��ˮһ����ɹ۲�H2S����������S����������һ����ɾ�����������SO2���Է�SO2�����һ������ʯ��ˮ�ܽ�CaCO3�����Ź۲죻��ʯ��ˮǰ���װϡ��ˮϴ��װ����Ϊ�˱��ڹ۲�SO2�Ƿ�����һ��������ȫ��ѡϡƷ����Һ�۲���ɫ���ʴ�Ϊ��
| ʵ�鷽�� | ���ܵ��������Ӧ�Ľ��� |
| ���Թ����巢��װ���м��������Ĺ�����Ʒ��ϡ���ᣬ��װ�ú���������ͨ��ʢ��ϡƷ����Һ��Ũ��ˮ��ϡ��ˮ�ͳ���ʯ��ˮ���Թ�ϴ��װ�ã� | ��Ʒ����ɫ����ˮ��ɫ��dz�����ǣ�����ʯ��ˮ����ǣ������庬��SO2��CO2�Ļ������ |
| ��Ʒ�첻��ɫ����ˮ��ɫ�����dz�����л��dz��֣�����ʯ��ˮ����ǣ�������ΪH2S��CO2�Ļ������ |
��������1���������Ʊ��ʺ�������ΪNa2SO3��Na2SO4��Na2CO3��Na2S��Na2SO3��Na2SO4��Na2CO3֮�䲻��Ӧ��Na2S��Na2SO3��Na2CO3�����ᷴӦ�ֱ�õ�H2S��SO2��CO2��Na2SO4�������Ӧ����������������ǵ�һ���壬Ҳ�����ǻ�����壬��������岻��ΪSO2��H2S����Ϊ���߷�Ӧ������������ݴ˻ش����⣻
��2����ͨ��Ũ��ˮһ����ɹ۲�H2S����������S����������һ����ɾ�����������SO2���Է�SO2�����һ������ʯ��ˮ�ܽ�CaCO3�����Ź۲죻��ʯ��ˮǰ���װϡ��ˮϴ��װ����Ϊ�˱��ڹ۲�SO2�Ƿ�����һ��������ȫ��ѡϡƷ����Һ�۲���ɫ����
��3��������������ӵļ��鷽�����μ�BaCl2��Һ�����г�������˵������������ӣ�
������������Ҫ��������Ļ���������ʣ��ڼ�������ʱҪע��˳��ͬʱҪ��ȥ���ʣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д�
�����Ŀ
Ϊ��̽�����ƾ��壨Na2S?nH2O���ڿ����еı仯��ij��ȤС���ʵ�鲽�輰�����¼���£��ٽ��������ƾ������ڱ������ϣ���¶�ڿ����У����������Ʒ����ʢˮ���ձ��У���������ɫ������Һ����ȡ��������Һ������������У�������ų�������Һ���ֳ��壮
��1���������������¼�������ų�����ijɷֿ����ǣ�����д�����֣� ��
��2�������ų�����Ϊ��������Ļ�����壬��ʹ��Ʒ����Һ��Ũ��ˮ������ʯ��ˮ�����Լ����һ�����ʵ������û���������ɣ���Ҫд��ʵ�鷽��������ͽ��ۣ�
��3�����Ҫ�������ƾ����Ƿ����������������ƣ����Ҫ˵������ķ���������ͽ��ۣ�������ȤС��ʵ��Ļ��������ʵ�飩�� ��
��1���������������¼�������ų�����ijɷֿ����ǣ�����д�����֣�
��2�������ų�����Ϊ��������Ļ�����壬��ʹ��Ʒ����Һ��Ũ��ˮ������ʯ��ˮ�����Լ����һ�����ʵ������û���������ɣ���Ҫд��ʵ�鷽��������ͽ��ۣ�
| ʵ�鷽�� | ���ܵ��������Ӧ�Ľ��� |
Ϊ��̽�����ƾ����ڿ����еı仯��ij��ȤС���ʵ�鲽�輰�����¼���£��ٽ��������ƾ������ڱ������ϣ���¶�ڿ����У����������Ʒ����ʢˮ���ձ��У���������ɫ������Һ����ȡ��������Һ������������У�������ų�������Һ���ֳ��壮
��1���������������¼���Էų�������в��룺
����٣�������H2S�� ����ڣ�������SO2�� ����ۿ�����CO2��
����ܣ�������______�� ����ݣ�������______��
��2�����ų�����������������Ļ���������У�Ũ��ϡ2��Ʒ����Һ��Ũ��ϡ2����ˮ������ʯ��ˮ��Ũ��ϡ2��������Һ���Թ����巢��װ�ã�ϴ��ƿ���Թ�ϴ��װ�ã������һ�����ʵ��������������ɣ����±��м�Ҫд��ʵ�鷽��������ͽ��ۣ�
��3�����Ҫ�������ƾ����Ƿ����������������ƣ����Ҫ˵������ķ���������ͽ��ۣ�______��
��1���������������¼���Էų�������в��룺
����٣�������H2S�� ����ڣ�������SO2�� ����ۿ�����CO2��
����ܣ�������______�� ����ݣ�������______��
��2�����ų�����������������Ļ���������У�Ũ��ϡ2��Ʒ����Һ��Ũ��ϡ2����ˮ������ʯ��ˮ��Ũ��ϡ2��������Һ���Թ����巢��װ�ã�ϴ��ƿ���Թ�ϴ��װ�ã������һ�����ʵ��������������ɣ����±��м�Ҫд��ʵ�鷽��������ͽ��ۣ�
| ʵ�鷽�� | ���ܵ��������Ӧ�Ľ��� |
| ______ | ______ |
| ______ |