��Ŀ����
��25�桢101kPa�£�1g�״�ȼ������CO2��Һ̬ˮʱ����22.68kJ�������Ȼ�ѧ����ʽ��ȷ����
| A��CH3OH��l��+3/2O2��g��===CO2��g��+2H2O��l����H=��725.8 kJ/mol |
| B��2CH3OH��l��+3O2��g��===2CO2��g��+4H2O��l����H=��1452 kJ/mol |
| C��2CH3OH��l��+3O2��g��===2CO2��g��+4H2O��l����H=��725.8 kJ/mol |
| D��2CH3OH��l��+3O2��g��===2CO2��g��+4H2O��l����H=��1452 kJ/mol |
B
�ų��������Ȼ�ѧ����ʽ���ԡ�������ʾ���ų�AD
�����������¿����1 mol�״���ȫȼ�շų�������Ϊ22.68kJ��32="725.8" kJ����2 mol�״���ȫȼ�շų�������Ϊ1452 kJ����ѡ��B��������
�����������¿����1 mol�״���ȫȼ�շų�������Ϊ22.68kJ��32="725.8" kJ����2 mol�״���ȫȼ�շų�������Ϊ1452 kJ����ѡ��B��������

��ϰ��ϵ�д�
�����Ŀ
 ���һ����ѧ��Ӧ����Ӧ����������������������������ͼ��ʾ�Ĺ�ϵ����÷�Ӧ����__________��Ӧ��
���һ����ѧ��Ӧ����Ӧ����������������������������ͼ��ʾ�Ĺ�ϵ����÷�Ӧ����__________��Ӧ��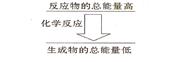

 2H2(g)+O2(g)�����Զ����������Ǹ����о�����Ҫ�ȵ㡣����Ϊ�����о�����������
2H2(g)+O2(g)�����Զ����������Ǹ����о�����Ҫ�ȵ㡣����Ϊ�����о����������� 