��Ŀ����
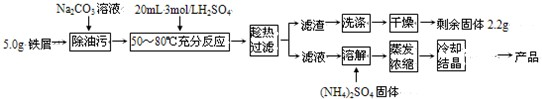 ��������茶���[��NH4��2Fe��SO4��2?6H2O]�ڿ����б�һ���������ȶ����Ƿ�����ѧ�г����Ļ�ԭ������ʵ�����Ʊ��������£�
��������茶���[��NH4��2Fe��SO4��2?6H2O]�ڿ����б�һ���������ȶ����Ƿ�����ѧ�г����Ļ�ԭ������ʵ�����Ʊ��������£�
�ش��������⣺
��1��Na2CO3��Һ���Գ����ۣ�ԭ���ǣ������ӷ���ʽ��ʾ��______��
��2���ж���м�����������Ӧ��ȫ��������______��
��3��ijͬѧ��Ϊ��м�����ᷴӦ���辫ȷ�¶ȣ���˲�ʹ����ˮԡ���ȣ����þƾ��ƶ϶���������ά���¶ȣ��������IJ���֮����______��
��4�������ȹ��ˡ������Һ��ʱ���Ի�ɫ��������ԭ���ǣ������ӷ���ʽ��ʾ��______��
��5������������������ʵ����Ӧ������NH4��2SO4���������Ϊ______g��
��6���������������ڴ�������������ȴ�ᾧ�����ĸҺ��Ѵ���������______��
�⣺��1��Na2CO3��Һ��CO32-ˮ��CO32-+H2O HCO3-+OH-��ʹNa2CO3��Һ�ʼ��ԣ����Լ��Ե�Na2CO3��Һϴȥ�������ۣ�
HCO3-+OH-��ʹNa2CO3��Һ�ʼ��ԣ����Լ��Ե�Na2CO3��Һϴȥ�������ۣ�
�ʴ�Ϊ��CO32-+H2O HCO3-+OH-��
HCO3-+OH-��
��2�����������ݲ�������ֻ�м��������ݲ�����ʱ��˵����м�����������Ӧ��ȫ��
�ʴ�Ϊ�����������ݲ�������ֻ�м��������ݲ�������
��3�������Ʋ�ȷ�¶ȹ���Fe2+�ױ��������������������ɣ�������Ȳ���ȫ��������ȼ�գ�������Σ�գ�
�ʴ�Ϊ�������Ʋ�ȷ�¶ȹ���Fe2+�ױ��������������������ɣ�������Ȳ���ȫ��
��4��Fe2+�ױ���������ΪFe3+����Ӧ���ӷ���ʽΪ��4Fe2++O2+4H+=4Fe3++2H2O��
�ʴ�Ϊ��4Fe2++O2+4H+=4Fe3++2H2O��
��5���ɹ������̿�֪��5g��м�к��в���������2.2g��������������Ϊ5g-2.2g=2.8g�����ʵ���Ϊ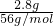 =0.05mol������������茶���[��NH4��2Fe��SO4��2?6H2O]��֪����Ҫ0.05mol��NH4��2SO4�����Գ�����NH4��2SO4��������Ϊ0.05mol��132g/mol=6.6g��
=0.05mol������������茶���[��NH4��2Fe��SO4��2?6H2O]��֪����Ҫ0.05mol��NH4��2SO4�����Գ�����NH4��2SO4��������Ϊ0.05mol��132g/mol=6.6g��
�ʴ�Ϊ��6.6
��6����ȴ�ᾧ���ĸҺ�к���FeSO4����NH4��2SO4��Ҫ�������ԭ�ϣ����ԡ���ȴ�ᾧ�����ĸҺ��Ѵ���������ѭ��ʹ�ã�
�ʴ�Ϊ��ѭ��ʹ�ã�
��������1��Na2CO3��Һ��CO32-ˮ��ʹNa2CO3��Һ�ʼ��ԣ����Լ��Ե�Na2CO3��Һϴȥ����м���������ۣ�
��2�����������ݲ�������ֻ�м��������ݲ�����ʱ����м�����������Ӧ��ȫ��
��3���¶ȹ���Fe2+�ױ��������������������ɣ�������Ȳ���ȫ��
��4��Fe2+�ױ���������ΪFe3+��
��5���ɹ������̿�֪��5g��м�к��в���������2.2g��������������Ϊ2.8g�����ʵ���Ϊ0.05mol������������茶���[��NH4��2Fe��SO4��2?6H2O]��֪����Ҫ0.05mol��NH4��2SO4������m=nM���㣨NH4��2SO4����������
��6����ȴ�ᾧ���ĸҺ�к���FeSO4����NH4��2SO4��Ҫ�������ԭ�ϣ�
���������⿼������ˮ�⡢�������ʡ����ʷ����ᴿ���й�ʵ�������ʵ���������ơ���ѧ�����Լ��ۺ�����֪ʶ�������������ȣ���Ŀ�Ѷ��еȣ�ע�����֪ʶ��ȫ�����գ�
 HCO3-+OH-��ʹNa2CO3��Һ�ʼ��ԣ����Լ��Ե�Na2CO3��Һϴȥ�������ۣ�
HCO3-+OH-��ʹNa2CO3��Һ�ʼ��ԣ����Լ��Ե�Na2CO3��Һϴȥ�������ۣ��ʴ�Ϊ��CO32-+H2O
 HCO3-+OH-��
HCO3-+OH-����2�����������ݲ�������ֻ�м��������ݲ�����ʱ��˵����м�����������Ӧ��ȫ��
�ʴ�Ϊ�����������ݲ�������ֻ�м��������ݲ�������
��3�������Ʋ�ȷ�¶ȹ���Fe2+�ױ��������������������ɣ�������Ȳ���ȫ��������ȼ�գ�������Σ�գ�
�ʴ�Ϊ�������Ʋ�ȷ�¶ȹ���Fe2+�ױ��������������������ɣ�������Ȳ���ȫ��
��4��Fe2+�ױ���������ΪFe3+����Ӧ���ӷ���ʽΪ��4Fe2++O2+4H+=4Fe3++2H2O��
�ʴ�Ϊ��4Fe2++O2+4H+=4Fe3++2H2O��
��5���ɹ������̿�֪��5g��м�к��в���������2.2g��������������Ϊ5g-2.2g=2.8g�����ʵ���Ϊ
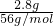 =0.05mol������������茶���[��NH4��2Fe��SO4��2?6H2O]��֪����Ҫ0.05mol��NH4��2SO4�����Գ�����NH4��2SO4��������Ϊ0.05mol��132g/mol=6.6g��
=0.05mol������������茶���[��NH4��2Fe��SO4��2?6H2O]��֪����Ҫ0.05mol��NH4��2SO4�����Գ�����NH4��2SO4��������Ϊ0.05mol��132g/mol=6.6g���ʴ�Ϊ��6.6
��6����ȴ�ᾧ���ĸҺ�к���FeSO4����NH4��2SO4��Ҫ�������ԭ�ϣ����ԡ���ȴ�ᾧ�����ĸҺ��Ѵ���������ѭ��ʹ�ã�
�ʴ�Ϊ��ѭ��ʹ�ã�
��������1��Na2CO3��Һ��CO32-ˮ��ʹNa2CO3��Һ�ʼ��ԣ����Լ��Ե�Na2CO3��Һϴȥ����м���������ۣ�
��2�����������ݲ�������ֻ�м��������ݲ�����ʱ����м�����������Ӧ��ȫ��
��3���¶ȹ���Fe2+�ױ��������������������ɣ�������Ȳ���ȫ��
��4��Fe2+�ױ���������ΪFe3+��
��5���ɹ������̿�֪��5g��м�к��в���������2.2g��������������Ϊ2.8g�����ʵ���Ϊ0.05mol������������茶���[��NH4��2Fe��SO4��2?6H2O]��֪����Ҫ0.05mol��NH4��2SO4������m=nM���㣨NH4��2SO4����������
��6����ȴ�ᾧ���ĸҺ�к���FeSO4����NH4��2SO4��Ҫ�������ԭ�ϣ�
���������⿼������ˮ�⡢�������ʡ����ʷ����ᴿ���й�ʵ�������ʵ���������ơ���ѧ�����Լ��ۺ�����֪ʶ�������������ȣ���Ŀ�Ѷ��еȣ�ע�����֪ʶ��ȫ�����գ�

��ϰ��ϵ�д�
 ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
�����Ŀ