��Ŀ����
��ʳ���г����и����ӡ�þ���ӡ���������ӵȿ����������⣬��������ɰ�Ȳ��������ʡ�����ʳ�õľ������ô�ʳ���ᴿ���õ��ġ�ͨ���̲��С����ε��ᴿ��ʵ��ش��������⡣
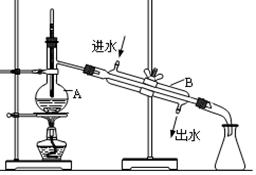
��1��ʵ���ҽ���NaCl��Һ����ʱ��һ�������²������̢ٷ��þƾ��ƣ��ڹ̶���Ȧλ�ã��۷�����������������ʢ��NaCl��Һ�����ܼ��Ƚ��裻��ֹͣ���ȡ�����ȷ�IJ���˳��Ϊ____________________________��
��2�������ܽ��Ҫ��ȥ�����������������������ij����Լ�������ij����Լ�����Ϊ���ѧʽ��______________��___________________��______________������һ��˳��Ȼ���ٽ��й��˵Ȳ�����
��3��д����2���л�ѧ��Ӧ����ʽ
____________________________��___________________________��
____________________________��____________________________��
��4������������������Ӻ�����ǰ��Һ������SO42�����ӣ�___________________________________________________________________________��
��1��ʵ���ҽ���NaCl��Һ����ʱ��һ�������²������̢ٷ��þƾ��ƣ��ڹ̶���Ȧλ�ã��۷�����������������ʢ��NaCl��Һ�����ܼ��Ƚ��裻��ֹͣ���ȡ�����ȷ�IJ���˳��Ϊ____________________________��
��2�������ܽ��Ҫ��ȥ�����������������������ij����Լ�������ij����Լ�����Ϊ���ѧʽ��______________��___________________��______________������һ��˳��Ȼ���ٽ��й��˵Ȳ�����
��3��д����2���л�ѧ��Ӧ����ʽ
____________________________��___________________________��
____________________________��____________________________��
��4������������������Ӻ�����ǰ��Һ������SO42�����ӣ�___________________________________________________________________________��
1���٢ڢۢܢ�(2��)
2��BaCl2 NaOH Na2CO3(��1��)
3��Na2SO4 + BaCl2 = BaSO4��+ 2NaCl ��Na2CO3 + BaCl2 = BaCO3��+2NaCl
2NaOH+ MgCl2 = Mg(OH)2��+2NaCl , Na2CO3 + CaCl2 = CaCO3��+2NaCl(��1��)
4��ȡ������Һ���Թ��еμӼ���BaCl2��Һ��������ְ�ɫ���ǻ����������Һ����SO42���������ֻ��Ǿ�˵��SO42���Ѿ�������(2��)
2��BaCl2 NaOH Na2CO3(��1��)
3��Na2SO4 + BaCl2 = BaSO4��+ 2NaCl ��Na2CO3 + BaCl2 = BaCO3��+2NaCl
2NaOH+ MgCl2 = Mg(OH)2��+2NaCl , Na2CO3 + CaCl2 = CaCO3��+2NaCl(��1��)
4��ȡ������Һ���Թ��еμӼ���BaCl2��Һ��������ְ�ɫ���ǻ����������Һ����SO42���������ֻ��Ǿ�˵��SO42���Ѿ�������(2��)
��1������װ���ԭ�������¶��ϣ��������ң�������ȷ��ʵ�����˳���Ǣ٢ڢۢܢݡ�
��2���������ʵķ�����ᴿ��Ca2����̼���Ƴ�ȥ��Mg2�����������Ƴ�ȥ��SO42�����Ȼ�����ȥ�������������ữ�������ڹ������Ȼ���Ҫ��̼��������������̼���Ʊ�������Ȼ����ĺ��棬���������ƿ������������
��3������ʵ����̿�֪����Ӧ�ķ���ʽ��Na2SO4 + BaCl2 = BaSO4��+ 2NaCl��Na2CO3 + BaCl2 = BaCO3��+2NaCl��2NaOH+ MgCl2 = Mg(OH)2��+2NaCl��Na2CO3 + CaCl2 = CaCO3��+2NaCl��
��4��Ҫ����SO42�����������Ȼ�����Һ����Ϊ��ֹ�������ӣ���CO32����SO32����Ag����)�ĸ��ţ���Ҫ���ȼ������ᣬȻ���ڼ����Ȼ�����Һ���������������֤SO42���Ĵ��ڵģ�ʵ����Բ��������ᡣȡ������Һ���Թ��еμӼ���BaCl2��Һ��������ְ�ɫ���ǻ����������Һ����SO42���������ֻ��Ǿ�˵��SO42���Ѿ�������
��2���������ʵķ�����ᴿ��Ca2����̼���Ƴ�ȥ��Mg2�����������Ƴ�ȥ��SO42�����Ȼ�����ȥ�������������ữ�������ڹ������Ȼ���Ҫ��̼��������������̼���Ʊ�������Ȼ����ĺ��棬���������ƿ������������
��3������ʵ����̿�֪����Ӧ�ķ���ʽ��Na2SO4 + BaCl2 = BaSO4��+ 2NaCl��Na2CO3 + BaCl2 = BaCO3��+2NaCl��2NaOH+ MgCl2 = Mg(OH)2��+2NaCl��Na2CO3 + CaCl2 = CaCO3��+2NaCl��
��4��Ҫ����SO42�����������Ȼ�����Һ����Ϊ��ֹ�������ӣ���CO32����SO32����Ag����)�ĸ��ţ���Ҫ���ȼ������ᣬȻ���ڼ����Ȼ�����Һ���������������֤SO42���Ĵ��ڵģ�ʵ����Բ��������ᡣȡ������Һ���Թ��еμӼ���BaCl2��Һ��������ְ�ɫ���ǻ����������Һ����SO42���������ֻ��Ǿ�˵��SO42���Ѿ�������

��ϰ��ϵ�д�
�����Ŀ