��Ŀ����
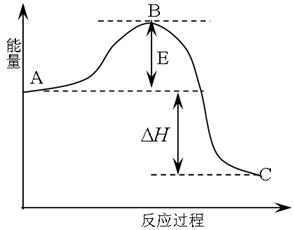
����14�֣� 2SO2(g)+O2(g) =2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2(g)����Ϊ1mol SO3�Ħ�H=-99kJ��mol-1.��ش��������⣺
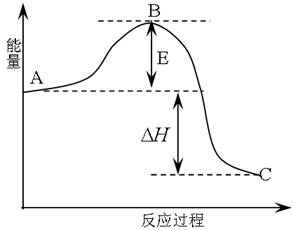
��1��ͼ��A��C�ֱ��ʾ �� ��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿�����Ӱ�족����Ӱ�족�� ���÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ� �������� ��
��2��ͼ�С�H= KJ��mol-1��
��3��V2O5�Ĵ�ѭ����������Ϊ��V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�����������д���ô�ѭ�������Ļ�ѧ����ʽ ��
��4�������Ӧ���ʦԣ�SO2��Ϊ0.05 mol��L-1��min-1,��ԣ�O2��= mol��L-1��min-1����(SO3)= mol��L-1��min-1��
��5����֪�������ȼ����Ϊ296 KJ��mol-1��������S(s)����3 molSO3(g)�ġ�H ��Ҫ�������̣���
����������1����Ӧ����������������������Ӱ�죬���ͣ���Ϊ�����ı��˷�Ӧ������ʹ���E���͡�����1�֣�
��2��-198����1�֣�
��3��SO2 +V2O5=SO3+2VO2 ��4VO2+ O2=2V2O5 ����4�֣�
��4��0.025 ��0.05 ������1�֣�
��5��S(s)+O2(g) =SO2(g) ��H1=-296 KJ��mol-1 ,
SO2(g)+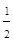 O2(g) =SO3(g)
��H2=-99
KJ��mol-1
O2(g) =SO3(g)
��H2=-99
KJ��mol-1
3 S(s)+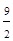 O2(g)=3SO3(g) ��H=3(��H1+��H2)=-1185
KJ��mol-1��2�֣�
O2(g)=3SO3(g) ��H=3(��H1+��H2)=-1185
KJ��mol-1��2�֣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�