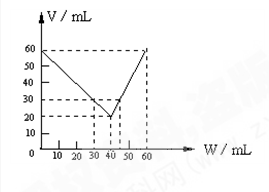
��Ŀ����
��6�֣���H2��C2H4��C2H2��ɵ�1L������壬��һ�������·����ӳɷ�Ӧ����������������XL����ش�����������ڱ�״���²ⶨ��
�ŵ���Ӧ�������������ʣ��ʱ��X��ȡֵ��Χ�� ��
������Ӧ�������������ʣ�࣬X=0.4ʱ��ԭ�������H2��C2H4��C2H2�������Ϊ ��
����X=0.6ʱ���ҷ�Ӧ���������ܶ�Ϊ0.714g/Lʱ��ԭ�������H2��C2H4��C2H2�����֮��Ϊ ��
�ŵ���Ӧ�������������ʣ��ʱ��X��ȡֵ��Χ�� ��
������Ӧ�������������ʣ�࣬X=0.4ʱ��ԭ�������H2��C2H4��C2H2�������Ϊ ��
����X=0.6ʱ���ҷ�Ӧ���������ܶ�Ϊ0.714g/Lʱ��ԭ�������H2��C2H4��C2H2�����֮��Ϊ ��
��1/3<X<1/2 ��3��1��1 ��7��2��1
��1����ϩ����Ȳ�����������ӳɷ�Ӧ�ķ���ʽ�ֱ���CH2=CH2��H2 CH3CH3��C2H2��2H2
CH3CH3��C2H2��2H2 CH3CH3�����ݷ���ʽ��֪�����ǡ������ϩ�������ӳɣ���X��0.5�����ǡ������Ȳ�������ӳɣ���X��1/3������X��ȡֵ��Χ��1/3<X<1/2��
CH3CH3�����ݷ���ʽ��֪�����ǡ������ϩ�������ӳɣ���X��0.5�����ǡ������Ȳ�������ӳɣ���X��1/3������X��ȡֵ��Χ��1/3<X<1/2��
��2����ԭ�������H2��C2H4��C2H2������ֱ���x��y��z������ݷ���ʽ��֪��y��2z��x��x��y��z��1��y��z��0.4�����Խ��x��0.6��y��0.2��z��0.2�����ԭ�������H2��C2H4��C2H2�������Ϊ3�U1�U1��
��3����Ӧ���������ܶ�Ϊ0.714g/L����������ƽ����Է���������0.714����22.4��16����˵������һ���ǹ����ġ���˷�Ӧ�������������������Ļ����������ߵ����֮����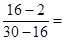 1�U1�����Է�Ӧ����0.3L������0.3L���顣��ԭ�������H2��C2H4��C2H2������ֱ���a��b��c����a��b��c��1��b��c��0.3������a��0.7������Ϊb��2c��0.7��0.3��0.4������c��0.1����b��0.2�����ԭ�������H2��C2H4��C2H2�������Ϊ7�U2�U1��
1�U1�����Է�Ӧ����0.3L������0.3L���顣��ԭ�������H2��C2H4��C2H2������ֱ���a��b��c����a��b��c��1��b��c��0.3������a��0.7������Ϊb��2c��0.7��0.3��0.4������c��0.1����b��0.2�����ԭ�������H2��C2H4��C2H2�������Ϊ7�U2�U1��
 CH3CH3��C2H2��2H2
CH3CH3��C2H2��2H2 CH3CH3�����ݷ���ʽ��֪�����ǡ������ϩ�������ӳɣ���X��0.5�����ǡ������Ȳ�������ӳɣ���X��1/3������X��ȡֵ��Χ��1/3<X<1/2��
CH3CH3�����ݷ���ʽ��֪�����ǡ������ϩ�������ӳɣ���X��0.5�����ǡ������Ȳ�������ӳɣ���X��1/3������X��ȡֵ��Χ��1/3<X<1/2����2����ԭ�������H2��C2H4��C2H2������ֱ���x��y��z������ݷ���ʽ��֪��y��2z��x��x��y��z��1��y��z��0.4�����Խ��x��0.6��y��0.2��z��0.2�����ԭ�������H2��C2H4��C2H2�������Ϊ3�U1�U1��
��3����Ӧ���������ܶ�Ϊ0.714g/L����������ƽ����Է���������0.714����22.4��16����˵������һ���ǹ����ġ���˷�Ӧ�������������������Ļ����������ߵ����֮����
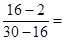 1�U1�����Է�Ӧ����0.3L������0.3L���顣��ԭ�������H2��C2H4��C2H2������ֱ���a��b��c����a��b��c��1��b��c��0.3������a��0.7������Ϊb��2c��0.7��0.3��0.4������c��0.1����b��0.2�����ԭ�������H2��C2H4��C2H2�������Ϊ7�U2�U1��
1�U1�����Է�Ӧ����0.3L������0.3L���顣��ԭ�������H2��C2H4��C2H2������ֱ���a��b��c����a��b��c��1��b��c��0.3������a��0.7������Ϊb��2c��0.7��0.3��0.4������c��0.1����b��0.2�����ԭ�������H2��C2H4��C2H2�������Ϊ7�U2�U1��
��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
�����Ŀ