��Ŀ����
ij�����ķ�ˮ�к���FeSO4��H2SO4��Ag2SO4��Al2��SO4��3��һЩ���ࡣij�о���ѧϰ������ⶨ�˷�ˮ�и����ʵĺ������������ܽ�����ݣ����б�����
��һ��ˮ�и����ʵĺ���
|
���� |
FeSO4 |
H2SO4 |
Ag2SO4 |
Al2(SO4)2 |
���� |
|
���������������� |
15.0 |
7.0 |
0.40 |
0.34 |
5.0 |
���� FeSO4��Al2��SO4��3��ˮ�е��ܽ��
|
�¶�/�� |
0 |
10 |
20 |
30 |
40 |
50 |
|
FeSO4��Һ�ȣ�g�� |
15.6 |
20.5 |
26.5 |
32.9 |
40.2 |
48.6 |
|
Al2(SO4)3�ܽ�ȣ�g�� |
31.2 |
33.5 |
36.4 |
40.4 |
45.7 |
52.2 |
�ÿ�������ݱ������ݣ��������ˮ���������������øó��ķ���м����������ߣ����ռ���Һ�����ᴦ������ˮ������FeSO4��7H2O��Ag��
��1������д���пհף���ɵõ�Ag��ʵ�鷽����
�ٽ�������ߵķ���м�Ⱥ����ȵ��ռ���Һ����ˮ����ϴ�ӣ�Ŀ���� ��
�ڽ�������ˮ���ˣ�������ˮϴ��������ϴ��Һ������Һ�������ã�
�� ��Ŀ����ʹAg+ȫ����ԭΪ����Ag��
�� ��Ŀ���Ƿ����Ag��
��2����д�������IJ��裬��ȥAl3+���õ���Ҫ�ɷ�ΪFeSO4��7H2O���塣
�ݽ��� ����ڢܲ�������Һ��Ϻ����������������Һ��pHΪ3-4�� ���˳�FeSO4��7H2O����
��3��д������������л�ѧ��Ӧ�����ӷ���ʽ ��
��4���ڲ�����У����������������pH��Ŀ���� ��
��1���١�ϴȥ���ۡ���1�֣� �ۡ��ڢڵ���Һ�м����Թ�����ϴ�ӹ��ķ���м��1�֣���
��ַ�Ӧ����ˣ�1�֣�
�ܡ����۵���������������ϡ���ᣨ1�֣������ˣ���Һ�������ã�1�֣�
��2���ݢ� ��2�֣�����Һ���ȣ��¶Ȳ�����80�棩����Ũ����1�֣�����ȴ�ᾧ��1�֣�
��3�� ������
������ ��
��
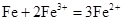 ������
������ ��4�֣�
��4�֣�
��4������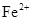 ��ˮ�⣨��ֹ
��ˮ�⣨��ֹ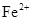 ����������2�֣�
����������2�֣�
����������

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д�ij�����ķ�ˮ�к���FeSO4��H2SO4��Ag2SO4��Al2��SO4��3��һЩ���ࡣij�о���ѧϰ������ⶨ�˷�ˮ�и����ʵĺ������������ܽ�����ݣ����б�����
��һ��ˮ�и����ʵĺ���
| ���� | FeSO4 | H2SO4 | Ag2SO4 | Al2(SO4)2 | ���� |
| ���������������� | 15.0 | 7.0 | 0.40 | 0.34 | 5.0 |
���� FeSO4��Al2��SO4��3��ˮ�е��ܽ��
| �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 |
| FeSO4��Һ�ȣ�g�� | 15.6 | 20.5 | 26.5 | 32.9 | 40.2 | 48.6 |
| Al2(SO4)3�ܽ�ȣ�g�� | 31.2 | 33.5 | 36.4 | 40.4 | 45.7 | 52.2 |
�ÿ�������ݱ������ݣ��������ˮ���������������øó��ķ���м����������ߣ����ռ���Һ�����ᴦ������ˮ������FeSO4��7H2O��Ag��
��1������д���пհף���ɵõ�Ag��ʵ�鷽����
�ٽ�������ߵķ���м�Ⱥ����ȵ��ռ���Һ����ˮ����ϴ�ӣ�Ŀ���� ��
�ڽ�������ˮ���ˣ�������ˮϴ��������ϴ��Һ������Һ�������ã�
�� ��Ŀ����ʹAg+ȫ����ԭΪ����Ag��
�� ��Ŀ���Ƿ����Ag��
��2����д�������IJ��裬��ȥAl3+���õ���Ҫ�ɷ�ΪFeSO4��7H2O���塣
�ݽ��� ����ڢܲ�������Һ��Ϻ����������������Һ��pHΪ3-4�� ���˳�FeSO4��7H2O����
��3��д������������л�ѧ��Ӧ�����ӷ���ʽ ��
��4���ڲ�����У����������������pH��Ŀ���� ��
ij�����ķ�ˮ�к���FeSO4��H2SO4��Ag2SO4��Al2��SO4��3��һЩ���ࡣij�о���ѧϰ������ⶨ�˷�ˮ�и����ʵĺ������������ܽ�����ݣ����б�����
��һ��ˮ�и����ʵĺ���
| ���� | FeSO4 | H2SO4 | Ag2SO4 | Al2(SO4)2 | ���� |
| ���������������� | 15.0 | 7.0 | 0.40 | 0.34 | 5.0 |
| �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 |
| FeSO4��Һ�ȣ�g�� | 15.6 | 20.5 | 26.5 | 32.9 | 40.2 | 48.6 |
| Al2(SO4)3�ܽ�ȣ�g�� | 31.2 | 33.5 | 36.4 | 40.4 | 45.7 | 52.2 |
��1������д���пհף���ɵõ�Ag��ʵ�鷽����
�ٽ�������ߵķ���м�Ⱥ����ȵ��ռ���Һ����ˮ����ϴ�ӣ�Ŀ���� ��
�ڽ�������ˮ���ˣ�������ˮϴ��������ϴ��Һ������Һ�������ã�
�� ��Ŀ����ʹAg+ȫ����ԭΪ����Ag��
�� ��Ŀ���Ƿ����Ag��
��2����д�������IJ��裬��ȥAl3+���õ���Ҫ�ɷ�ΪFeSO4��7H2O���塣
�ݽ��� ����ڢܲ�������Һ��Ϻ����������������Һ��pHΪ3-4�� ���˳�FeSO4��7H2O����
��3��д������������л�ѧ��Ӧ�����ӷ���ʽ ��
��4���ڲ�����У����������������pH��Ŀ���� ��