��Ŀ����
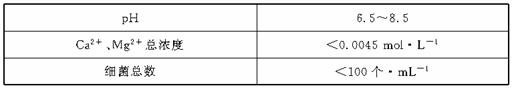
������ԭˮ����������ˮ�Ĺ�������ʾ��ͼ
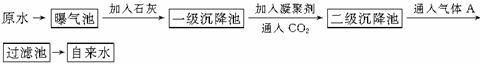
(1)ԭˮ�к�Ca2����Mg2����HCO
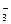 ��Cl���ȣ�����ʯ�Һ�����Ca(OH)2�������������ɸ��ֽⷴӦ��д������һ�����ӷ���ʽ_________________________________
��Cl���ȣ�����ʯ�Һ�����Ca(OH)2�������������ɸ��ֽⷴӦ��д������һ�����ӷ���ʽ_________________________________ (2)���ۼ���ȥ������������Ĺ��� (��д��ţ���ѡ����)
��ֻ���������� ��ֻ�ǻ�ѧ���� ���������ͻ�ѧ����FeSO4��7H2O�dz��õ����ۼ�������ˮ���������� ������
(3)ͨ�������̼��Ŀ���� �� ��
(4)����A�������� �����������ǻ�������A��ˮ��Ӧ�IJ������ �ԡ�
(5)���������У� ������Ϊ����A�Ĵ���Ʒ��(��д���)
��Ca(ClO)2 ��Ũ��ˮ ��K2FeO4 ��SO2
(1)HCO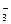 ��OH��===CO
��OH��===CO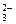 ��H2O
��H2O
Mg2����2OH��===Mg(OH)2��
Ca2����HCO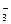 ��OH��===CaCO3����H2O
��OH��===CaCO3����H2O
��ѡ��һ������������Ҳ���֡�
(2)�� ��״Fe(OH)3
(3)��ȥCa2�� ����pH
(4)ɱ������ ǿ����
(5)�٢�
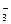 ��OH��===CO
��OH��===CO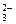 ��H2O
��H2OMg2����2OH��===Mg(OH)2��
Ca2����HCO
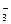 ��OH��===CaCO3����H2O
��OH��===CaCO3����H2O��ѡ��һ������������Ҳ���֡�
(2)�� ��״Fe(OH)3
(3)��ȥCa2�� ����pH
(4)ɱ������ ǿ����
(5)�٢�
(1)��Ca2����Mg2����HCO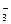 ��Cl����ˮ�м���Ca(OH)2�ɷ������ַ�Ӧ��HCO
��Cl����ˮ�м���Ca(OH)2�ɷ������ַ�Ӧ��HCO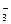 ��OH��===CO
��OH��===CO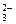 ��H2O��Mg2����2OH��===Mg(OH)2����Ca2����HCO
��H2O��Mg2����2OH��===Mg(OH)2����Ca2����HCO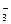 ��OH��===CaCO3����H2O�ȵȡ�
��OH��===CaCO3����H2O�ȵȡ�
(2)����FeSO4��7H2O Fe2��������ΪFe3������ˮ�����ɽ�״Fe(OH)3����ˮ�����ʶ�������
(3)ͨ��CO2Ŀ�ģ���ȥʣ��Ca(OH)2����Ca2����OH����
(4)����AӦΪCl2������ˮ��Ӧ���ɴ��������ǿ�����Զ�ɱ��������
(5)Ӧѡǿ��������
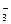 ��Cl����ˮ�м���Ca(OH)2�ɷ������ַ�Ӧ��HCO
��Cl����ˮ�м���Ca(OH)2�ɷ������ַ�Ӧ��HCO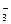 ��OH��===CO
��OH��===CO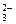 ��H2O��Mg2����2OH��===Mg(OH)2����Ca2����HCO
��H2O��Mg2����2OH��===Mg(OH)2����Ca2����HCO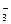 ��OH��===CaCO3����H2O�ȵȡ�
��OH��===CaCO3����H2O�ȵȡ�(2)����FeSO4��7H2O Fe2��������ΪFe3������ˮ�����ɽ�״Fe(OH)3����ˮ�����ʶ�������
(3)ͨ��CO2Ŀ�ģ���ȥʣ��Ca(OH)2����Ca2����OH����
(4)����AӦΪCl2������ˮ��Ӧ���ɴ��������ǿ�����Զ�ɱ��������
(5)Ӧѡǿ��������

��ϰ��ϵ�д�
�����Ŀ
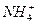 +
+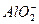 +2H2O
+2H2O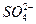
 H2S+2OH-
H2S+2OH- H2��+ Cu2+
H2��+ Cu2+ ��H��===H2O��CO2��
��H��===H2O��CO2��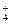 ��H2O
��H2O