��Ŀ����
ijͬѧ��̼������ͭƬ��ֱ����Դ��ϡ����Ϊԭ���ϣ�ʵ������ͨ�������²��ܷ����ķ�Ӧ��Cu+H2SO4��ϡ��=CuSO4+H2��
��1�������ұ߷����л����ܹ�ʵ����һ��Ӧ��װ��ͼ______��
��2�������ʵ��ʵ�ʲ���ʱ������װ�����Ӳ���������������ȫ��ͬ��
��ʵ������̼����ͭƬ�Ͼ�����ɫ���ݲ�������Һ��δ��Ϊ��ɫ��
���������ԭ��______��
�ڼ��谴�ո÷�������ʵ�飬���ռ���̼���ϲ����������Ϊ11.2L����״���£�����ù�����ͭƬ�Ϸ����ķ�ӦʽΪ______��
�۴�ʱҪʹ��Һ�ָ���ʵ��ǰ��״̬�������______��д�������ʵĻ�ѧʽ����������Ϊ______��

���𰸡���������1��ʵ�ָ÷�Ӧ��ѡ��CuΪ�����������Ϊ���
��2��̼����ͭƬ�Ͼ�����ɫ���ݲ�������Һ��δ��Ϊ��ɫ������ʵ��Ϊ���ˮ��C��Ϊ��������������Cu����������������һ��ʱ��ָ�ԭ״̬��Ӧ���������ˮ��
����⣺��1��������Cu+H2SO4��ϡ��=CuSO4+H2�������õ����ʵ�֣���CuΪ������CΪ�����������Ϊ���ᣬװ����ͼ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2����̼����ͭƬ�Ͼ�����ɫ���ݲ�������Һ��δ��Ϊ��ɫ������ʵ��Ϊ���ˮ�����������Դ���������ӷ��ˣ��ʴ�Ϊ���������Դ���������ӷ��ˣ�
��Cu�������������������ĵ缫��ӦΪ2H++2e-=H2�����ʴ�Ϊ��2H++2e-=H2����
��̼���ϲ����������Ϊ11.2LΪ�����������ʵ���Ϊ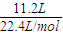 =0.5mol����2H2O
=0.5mol����2H2O O2��+2H2������Ҫ�ָ�ԭ������H2O������Ϊ0.5mol×2×18g/mol=18g��
O2��+2H2������Ҫ�ָ�ԭ������H2O������Ϊ0.5mol×2×18g/mol=18g��
�ʴ�Ϊ��H2O��18g��
���������⿼����ԭ������ȷ�缫���ϵ�ѡ���뷢���ĵ缫��Ӧ�ǽ����Ĺؼ���ע�����ʹ��Һ�ָ�ԭ����Ӧ��Ԫ�ؼ������غ�ĽǶ�����������Ŀ�ѶȲ���
��2��̼����ͭƬ�Ͼ�����ɫ���ݲ�������Һ��δ��Ϊ��ɫ������ʵ��Ϊ���ˮ��C��Ϊ��������������Cu����������������һ��ʱ��ָ�ԭ״̬��Ӧ���������ˮ��
����⣺��1��������Cu+H2SO4��ϡ��=CuSO4+H2�������õ����ʵ�֣���CuΪ������CΪ�����������Ϊ���ᣬװ����ͼ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��2����̼����ͭƬ�Ͼ�����ɫ���ݲ�������Һ��δ��Ϊ��ɫ������ʵ��Ϊ���ˮ�����������Դ���������ӷ��ˣ��ʴ�Ϊ���������Դ���������ӷ��ˣ�
��Cu�������������������ĵ缫��ӦΪ2H++2e-=H2�����ʴ�Ϊ��2H++2e-=H2����
��̼���ϲ����������Ϊ11.2LΪ�����������ʵ���Ϊ
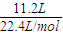 =0.5mol����2H2O
=0.5mol����2H2O O2��+2H2������Ҫ�ָ�ԭ������H2O������Ϊ0.5mol×2×18g/mol=18g��
O2��+2H2������Ҫ�ָ�ԭ������H2O������Ϊ0.5mol×2×18g/mol=18g���ʴ�Ϊ��H2O��18g��
���������⿼����ԭ������ȷ�缫���ϵ�ѡ���뷢���ĵ缫��Ӧ�ǽ����Ĺؼ���ע�����ʹ��Һ�ָ�ԭ����Ӧ��Ԫ�ؼ������غ�ĽǶ�����������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
 ijͬѧ��̼������ͭƬ��ֱ����Դ��ϡ����Ϊԭ���ϣ�ʵ������ͨ�������²��ܷ����ķ�Ӧ��
ijͬѧ��̼������ͭƬ��ֱ����Դ��ϡ����Ϊԭ���ϣ�ʵ������ͨ�������²��ܷ����ķ�Ӧ��

