��Ŀ����
��ʵ�����г��Բ����ƣ�Na2C2O4��Ϊ��Һ��ͨ��������ԭ�ζ����ⶨ���������Һ��Ũ�ȣ��䷴Ӧ�Ļ�ѧ����ʽΪ�� C2O42��+MnO4��+H+����Mn2++CO2��+H2O�������йز����Ƶζ��������ʵ�����������ȷ���� �� ��
| A���ζ������У�������ر������������Ʊ���ԭ |
| B������ʽ�ζ�����ȡ��������Һ |
| C���÷�Ӧ�У����ĵIJ������������ص����ʵ���֮��Ϊ5:2 |
| D��Ϊ���ڵζ��յ���жϣ��ζ�ʱ��������ϵ�м���ָʾ�� |
C
���������C����3����4�����������ı�����4��3����2��2��Mn����7����2������ԭ���ı�����7��2����1��5�����ݻ��ϼ�����������ȣ�������C2O42��ǰ��5��MnO4��ǰ��2���ٸ���Ԫ��ԭ���غ㣺C2O42��+2MnO4��+16H+===2Mn2++10CO2��+8H2O����������Һˮ��Ϊ������Һ�����ü�ʽ�ζ��ܣ����������Һ��������ɫ������ָʾ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
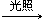 CH3CH2Cl+HCl
CH3CH2Cl+HCl CH3CH2Cl
CH3CH2Cl