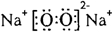��Ŀ����
�±��г��ˢ١�������Ԫ�������ڱ��е�λ�ã�
�밴Ҫ��ش��������⣺
��1��Ԫ�آٵ���̬�⻯��Ļ�ѧʽ��

��2��Ԫ�آ٣��ڵ�����������ˮ���������ǿ��Ϊ
��3��Ԫ�آݵ�ԭ�ӽṹʾ��ͼ��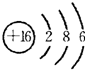

��4��������Ԫ���У�λ�ڵ���������ԭ�Ӱ뾶��С���ǣ���Ԫ�ط��ţ�
��5��������Ԫ�ص�����������У�����������������ǣ��ѧʽ��
��6����ʢ��ˮ��С�ձ��м���Ԫ�آ۵ĵ��ʣ�������Ӧ�����ӷ���ʽΪ��
��������Ӧ�����Һ���ټ���Ԫ�آܵĵ��ʣ�������Ӧ�����ӷ���ʽΪ��
| ��A | 0 | |||||||
| 1 | ��A | ��A | ��A | ��A | ��A | ��A | ||
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� |
��1��Ԫ�آٵ���̬�⻯��Ļ�ѧʽ��
CH4
CH4
Ԫ�آڵĵ��ʵ���ʽ��

��2��Ԫ�آ٣��ڵ�����������ˮ���������ǿ��Ϊ
HNO3
HNO3
��H2CO3
H2CO3
���ѧʽ����3��Ԫ�آݵ�ԭ�ӽṹʾ��ͼ��


��4��������Ԫ���У�λ�ڵ���������ԭ�Ӱ뾶��С���ǣ���Ԫ�ط��ţ�
Cl
Cl
��5��������Ԫ�ص�����������У�����������������ǣ��ѧʽ��
Al2O3
Al2O3
��6����ʢ��ˮ��С�ձ��м���Ԫ�آ۵ĵ��ʣ�������Ӧ�����ӷ���ʽΪ��
2Na+2H2O=2Na++2OH-+H2��
2Na+2H2O=2Na++2OH-+H2��
��������Ӧ�����Һ���ټ���Ԫ�آܵĵ��ʣ�������Ӧ�����ӷ���ʽΪ��
2Al+2H2O+2OH-=2AlO2-+3H2��
2Al+2H2O+2OH-=2AlO2-+3H2��
������������Ԫ�����ڱ�֪������C������N������Na������Al������S������ClԪ�أ�
��1��Ԫ�آٵ���̬�⻯���Ǽ��飬Ԫ�آڵĵ����ǵ�����
��2��ͬһ����Ԫ�أ�Ԫ�صķǽ���������ԭ���������������ǿ��Ԫ�صķǽ�����Խǿ������������ˮ��������Խǿ��
��3��Ԫ�آ���SԪ�أ���ԭ�Ӻ�����16�����ӣ���3�����Ӳ㣬�������6�����ӣ�
��4��ͬһ�����У�Ԫ�ص�ԭ�Ӱ뾶����ԭ���������������С��
��5��������Ԫ�ص�����������У��������������������������
��6���ƺ�ˮ��Ӧ�����������ƺ���������������������Һ��Ӧ����ƫ�����ƺ�������
��1��Ԫ�آٵ���̬�⻯���Ǽ��飬Ԫ�آڵĵ����ǵ�����
��2��ͬһ����Ԫ�أ�Ԫ�صķǽ���������ԭ���������������ǿ��Ԫ�صķǽ�����Խǿ������������ˮ��������Խǿ��
��3��Ԫ�آ���SԪ�أ���ԭ�Ӻ�����16�����ӣ���3�����Ӳ㣬�������6�����ӣ�
��4��ͬһ�����У�Ԫ�ص�ԭ�Ӱ뾶����ԭ���������������С��
��5��������Ԫ�ص�����������У��������������������������
��6���ƺ�ˮ��Ӧ�����������ƺ���������������������Һ��Ӧ����ƫ�����ƺ�������
����⣺����Ԫ�����ڱ�֪������C������N������Na������Al������S������ClԪ�أ�
��1��Ԫ�آٵ���̬�⻯���Ǽ��飬Ԫ�آڵĵ����ǵ��������������е�ԭ�Ӽ���ڹ������������������ʽΪ ��
��
�ʴ�Ϊ��CH4�� ��
��
��2��ͬһ����Ԫ�أ�Ԫ�صķǽ���������ԭ���������������ǿ��Ԫ�صķǽ�����Խǿ������������ˮ��������Խǿ��NԪ�صķǽ����Դ���CԪ�أ�������������Դ���̼�ᣬ�ʴ�Ϊ������
��3��Ԫ�آ���SԪ�أ���ԭ�Ӻ�����16�����ӣ���3�����Ӳ㣬�������6�����ӣ�������ԭ�ӽṹʾ��ͼΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4��ͬһ�����У�Ԫ�ص�ԭ�Ӱ뾶����ԭ���������������С�����Ե���������ԭ�Ӱ뾶��С��Ԫ������Ԫ�أ��ʴ�Ϊ��Cl��
��5��������Ԫ�ص�����������У���������������������������仯ѧʽΪAl2O3���ʴ�Ϊ��Al2O3��
��6���ƺ�ˮ��Ӧ�����������ƺ������������ӷ���ʽΪ��2Na+2H2O=2Na++2OH-+H2������������������Һ��Ӧ����ƫ�����ƺ���������Ӧ����ʽΪ��2Al+2H2O+2OH-=2AlO2-+3H2����
�ʴ�Ϊ��2Na+2H2O=2Na++2OH-+H2����2Al+2H2O+2OH-=2AlO2-+3H2����
��1��Ԫ�آٵ���̬�⻯���Ǽ��飬Ԫ�آڵĵ����ǵ��������������е�ԭ�Ӽ���ڹ������������������ʽΪ
 ��
���ʴ�Ϊ��CH4��
 ��
�� ��2��ͬһ����Ԫ�أ�Ԫ�صķǽ���������ԭ���������������ǿ��Ԫ�صķǽ�����Խǿ������������ˮ��������Խǿ��NԪ�صķǽ����Դ���CԪ�أ�������������Դ���̼�ᣬ�ʴ�Ϊ������
��3��Ԫ�آ���SԪ�أ���ԭ�Ӻ�����16�����ӣ���3�����Ӳ㣬�������6�����ӣ�������ԭ�ӽṹʾ��ͼΪ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����4��ͬһ�����У�Ԫ�ص�ԭ�Ӱ뾶����ԭ���������������С�����Ե���������ԭ�Ӱ뾶��С��Ԫ������Ԫ�أ��ʴ�Ϊ��Cl��
��5��������Ԫ�ص�����������У���������������������������仯ѧʽΪAl2O3���ʴ�Ϊ��Al2O3��
��6���ƺ�ˮ��Ӧ�����������ƺ������������ӷ���ʽΪ��2Na+2H2O=2Na++2OH-+H2������������������Һ��Ӧ����ƫ�����ƺ���������Ӧ����ʽΪ��2Al+2H2O+2OH-=2AlO2-+3H2����
�ʴ�Ϊ��2Na+2H2O=2Na++2OH-+H2����2Al+2H2O+2OH-=2AlO2-+3H2����
���������⿼����Ԫ��λ�á��ṹ�����ʵĹ�ϵ��Ԫ�ص��жϽϼ�ƫ��������ӵ���д�׳�����ע�������ʽ�к���3�����õ��Ӷԣ���ÿ����ԭ�Ӻ���һ���µ��Ӷԣ�

��ϰ��ϵ�д�
�����Ŀ

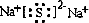
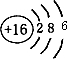
 ��A��B��C��D��E��F����Ԫ�أ���֪������λ��������ͬ�����ڣ��˵������������A��E��B��F�ֱ�ͬ���壻A��E�ֱ�����D��ԭ�Ӹ�����1�s1��2�sl�γɻ����B��C�ֱ�����D��ԭ�Ӹ�����1�s1��1�s2�γɻ����
��A��B��C��D��E��F����Ԫ�أ���֪������λ��������ͬ�����ڣ��˵������������A��E��B��F�ֱ�ͬ���壻A��E�ֱ�����D��ԭ�Ӹ�����1�s1��2�sl�γɻ����B��C�ֱ�����D��ԭ�Ӹ�����1�s1��1�s2�γɻ����