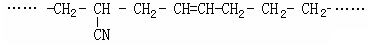题目内容
(1)写出下列原子的电子排布式:
①S________;②Cu________;③Mn________;④Se________;⑤Cr________;⑥I________;
(2)写出下列离子的排布式:①S2-________; ②Fe2+________;
①S________;②Cu________;③Mn________;④Se________;⑤Cr________;⑥I________;
(2)写出下列离子的排布式:①S2-________; ②Fe2+________;
(1)①[Ne]3s23p4;②[Ar]3d104s1;③[Ar]3d54s2;④[Ar]3d104s24p4;⑤[Ar]3d54s1;⑥[Kr]4d105s25p5
(2)①1s22s22p63s23p6;②[Ar]3d6
(2)①1s22s22p63s23p6;②[Ar]3d6

练习册系列答案
相关题目
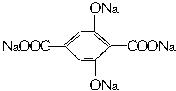
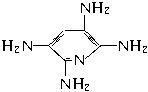
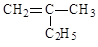 _____▲ ______
CH3CH2CH2COOH __ ▲ _______
_____▲ ______
CH3CH2CH2COOH __ ▲ _______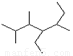 的名称
▲
的名称
▲