��Ŀ����
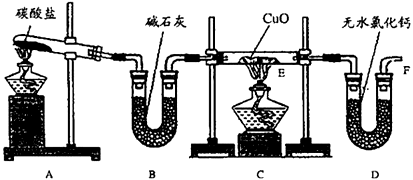
��10�֣�ʵ��������������NH3�ڼ��ȵ���������CuO��ַ�Ӧ����Cu��N2��H2O�����ⶨCu�����ԭ��������װ��ͼ���£�

��1�����Ӻ�װ�ú����װ�������Եķ�����
��
��2����װ��A��B��ȡ����������İ��������Թ���̼���εĻ�ѧʽΪ�� ��װ��B�м�ʯ�ҵ������ǣ� ��
��3��ʵ���й۲쵽Cװ�õ�E���в����������У� ��E���з�����Ӧ�Ļ�ѧ����ʽΪ�� ��
��4����ʵ���в�����������ݣ�
�ٿ�E�ܵ�����a��
��ʵ��ǰE�ܺ�CuO��������b��
�۳�ַ�Ӧ��E�ܺ�Cu�۵�������c����ȴ�����£�������������
�ܳ�ַ�Ӧ��D�ܼ���ʢ���ʵ�������d
��ѡ������������г�����Cu�����ԭ�������ļ���ʽ����Cu�⣬�����漰����Ԫ�ص����ԭ��������Ϊ��֪����Ar(Cu)== ��
��1����F�����ӵ��ܣ��������ܿڽ�û��ʢ��ˮ���ձ��У����Թ�A�����ܿ�������ð����ֹͣ���Ⱥ���ˮ�����뵼�����γ�һС��ˮ����˵��װ�õ����������á���3�֣�
��2�� ��NH4��2CO3 ��NH4HCO3 ��1�֣� ����ˮ�ֺͶ�����̼ ��1�֣�
��3����ɫ�����ɺ�ɫ��������ˮ����֣�1�֣�
2 NH3 + 3 CuO ![]() 3 Cu + N2 + 3 H2O ��2�֣�
3 Cu + N2 + 3 H2O ��2�֣�
��4��![]() ��2�֣�
��2�֣�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�