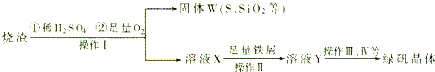��Ŀ����
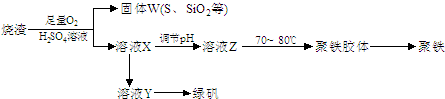
ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)���������£�
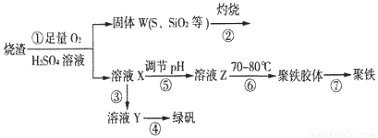
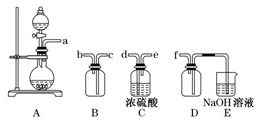
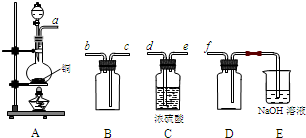
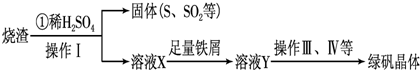
��1�������̢��еIJ���������ͨ��������Һ�У���Һ����ɫ����__________��
A��Ʒ����Һ??????? B����ɫʯ����Һ????? C������KMnO4��Һ???? D����ˮ
��2�����̢��У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ��___________________________________��
��3�����̢��У�������������___________________________��
��4�����̢��У������ᾧ��Ҫʹ�þƾ��ơ����Ǽܡ������ǣ�����Ҫ��������_______________��
��5�����̢ݵ���pH��ѡ�������Լ��е�___________ (��ѡ�����)��
A��ϡ����??????? B��CaCO3?????? C��NaOH��Һ
��6�����̢��У�����ҺZ���ȵ�70һ80����Ŀ����_____________________��
��7��ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣���÷�����ƽ��ȡ2.70g��Ʒ���ڽ���Ʒ�����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495g�����þ�����Ҫ�ɷ�Ϊ[(Fe(OH)(SO4)]n����þ�����Ʒ����Ԫ�ص���������Ϊ___________��(���������в�����Ԫ�غ���Ԫ��)��
��1��ACD ��2��4FeS + 3O2 + 6H2SO4 = 2Fe2(SO4)3 + 6H2O+4S ��3��Fe(����)
��4������������ ��5��C? ��6���ٽ�Fe3+��ˮ�� ��7��31.11%
��������
�����������1������W�к���S������ʱ�����SO2���塣A����SO2ͨ��Ʒ����Һ SO2��Ư���ԣ���ʹƷ����Һ��ɫ����ȷ��B��SO2��ˮ��Һ�����ԣ���ɫʯ����Һ��Ϊ��ɫ������C��SO2�л�ԭ�ԣ��ܱ�����KMnO4��Һ����Ϊ���ᣬKMnO4��ԭΪ��ɫ��MnSO4 .��ȷ�� D��SO2�л�ԭ�ԣ��ܱ���ˮ����Ϊ���ᣬ��ˮ����ԭΪ��ɫ��HBr����ȷ�����ѡ��ΪACD����2�����ݵ����غ�������غ㶨�ɿɵó��������У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ��4FeS+3O2+ 6H2SO4 = 2Fe2(SO4)3 +6H2O + 4S����3������ҺX�к���Fe2(SO4)3 ��H2SO4�����̷��������������塣���Թ������У������������ǻ�ԭ��Fe�ۡ���4�� ������ԭFe�ۻ�ԭ��Fe2(SO4)3 ��H2SO4��ҺΪFeSO4.Ҫ�Ӹ���Һ�еõ��̷�����Ϊ�����������ܽ�����¶ȵ�Ӱ��仯�ϴ����Բ�����ȴ�ȱ�����Һ�ķ�����ȡ��ʵ�������������Ũ������ȴ���ᾧ���ڹ������У������ᾧ��Ҫʹ�þƾ��ơ����Ǽܡ������ǣ�����Ҫ���������� ����5������������pH��ѡ�������Լ��е�Ӧ���ü��ȶ���NaOH���ܽ�ȴ���������Na+��Ca2+���׳�ȥ��ѡ��ΪC����6�� Fe2(SO4)3��ǿ�������Σ�ˮ�����Fe(OH)3���塣�ε�ˮ�ⷴӦ�����ȷ�Ӧ���ڹ������У�����ҺZ���ȵ�70һ80����Ŀ����Ϊ�˴ٽ�Fe3+��ˮ�� ����7���þ����ھ������ܽ����������Ȼ�����Һ���õ��ij���ΪBa SO4��n(Ba SO4)= 3.495g��233g/mol=0.015mol.����n(Fe)=0.015mol.m(Fe)=0.015mol��56g/mol=0.84g��������ھ�����FeԪ�صĺ���Ϊ(0.84g��2.70g) ��100% =33.11%��
���㣺����SO2�Ļ�ѧ���ʡ�����ʽ����д���ε�ˮ�⡢�����ķ��뷽����Ԫ�صĺ����ļ����֪ʶ��

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�