��Ŀ����
(7��) t��ʱ��ijNaOHϡ��Һ�У�c(H+)=10-amol/L��c(OH-)=10-bmol/L����֪a+b=12,��
��1�����¶��£�ˮ�����ӻ�����kw= ��
��2���ڸ��¶��£���100mL0.1mol/L��ϡ������100mL0.4mol/L��NaOH��Һ��Ϻ���Һ��pH= ��
3�����¶��£���100���pH1=a��ijǿ����Һ��1���pH2=b��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ�� ��
��4�����¶��£�pH=2��ij��HA��Һ��pH=10��NaOH��Һ�������Ϻ����Һ��pH=5���Է�����ԭ�� ��
��1��10-12 ��2��11 ��3��a+b = 14
��4��HAΪ���ᣬʹc(HA)>c(NaOH)����Ӧ����Һ������
����������1��ϡ��Һ�������Ӻ�OH����Ũ��֮����ˮ�����ӻ�������kw��c(H+)��c(OH-)��10-a��10-b��10��a��b��10��12��
��2���������ݿ��ж����������ǹ����ģ����Է�Ӧ��c(OH-)��
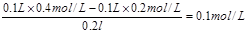 ��c(H+)��
��c(H+)��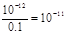 ������pH��11��
������pH��11��
��3����Ϻ���Һ�����ԣ��������ӵ����ʵ�����OH�������ʵ�����ȣ���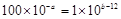 �����a��b��14��
�����a��b��14��
��4����HA��ǿ�ᣬ�����ǡ�÷�Ӧ����Һ�����ԡ������Һ��pH=5��˵����Һ�����ԣ����HAһ�������ᣬ����Ӧ��HA�ǹ����ģ�������Һ���������ԡ�
