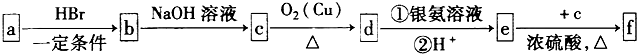��Ŀ����
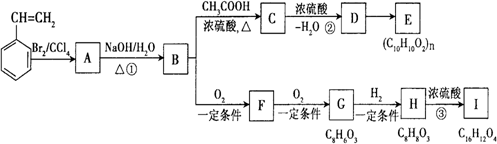
����ϩ��![]() ����A��B��C��D��E��F��G��H��I���л���֮���������ͼ��ʾת����ϵ��
����A��B��C��D��E��F��G��H��I���л���֮���������ͼ��ʾת����ϵ��
 ��ش��������⣺
��ش��������⣺
��1����Ӧ�١��ڵķ�Ӧ���ͷֱ�������������������������������������������������
��2��C���ܵĽṹ��ʽ��������������������������������������������������������
��3��H�ж���ͬ���칹�塣��������������ͬ���칹�干�������������֡�
����FeCl![]() ��Һ������ɫ��Ӧ��
��Һ������ɫ��Ӧ��
��lmolH�������3molNaOH��Ӧ��
�۱�����ֻ������ȡ������
���У�����һ��ͬ���칹��Ľṹ��ʽΪ ��
��4����Ӧ�۵Ļ�ѧ����ʽ�� ��
��5���۱���ϩ�������Ʊ���Ƶ��Ե���ϡ��ɱ���ϩ�ϳ����ָ߾���Ļ�ѧ����ʽΪ
����
��1��ˮ�ⷴӦ��ȡ����Ӧ������ȥ��Ӧ
��2��
��3��3
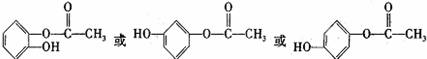
��4��
��5��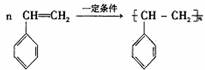

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
�����Ŀ