��Ŀ����
���ྻú�������о����������൱�ձ飬������Աͨ�������ú������¯�н�����������ˮ�����ķ�����������������ֵ�ߴ�122500~16000 kJ��m-3��ú̿��������Ҫ�ɷ���CO��H2��CO��H2����Ϊ��Դ�ͻ���ԭ�ϣ�Ӧ��ʮ�ֹ㷺��
��1����֪��C(s)+O2(g)=CO2(g) ��H1����393.5 kJ��mol-1 ��
2H2(g)+O2(g)=2H2O(g) ��H2����483.6 kJ��mol-1 ��
C(s)+H2O(g)=CO(g)+H2(g) ��H3����131.3 kJ��mol-1 ��
��ӦCO(g)+H2(g) +O2(g)= H2O(g)+CO2(g)����H= kJ��mol-1����״���µ�ú̿����CO��H2��33.6 L��������ȫ��Ӧ����CO2��H2O����Ӧ������ת�� mol e-��
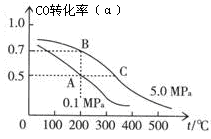
��2���ܱ������г���10 mol CO��20 mol H2���ڴ��������·�Ӧ���ɼ״���CO(g)+2H2(g)  CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��

����A��B�����ʾ��ijʱ�̴ﵽ��ƽ��״̬����ʱ��A��ʱ���������ΪVAL������¶��µ�ƽ�ⳣ��K= ��A��B����ʱ���������ʵ����ʵ���֮��Ϊn(A)����n(B)��= ��
����A��C���㶼��ʾ�ﵽ��ƽ��״̬�����Է�Ӧ��ʼ����ƽ��״̬�����ʱ��tA tC������ڡ�����С�ڡ����ڡ�����
���ڲ��ı䷴Ӧ������������£�Ϊ���CO��ת���ʿɲ�ȡ�Ĵ�ʩ�� ��
A.���� B.��ѹ C.ʹ�ô��� D.���״��ӻ����ϵ�з������
��1��-524.83kJ•mol-1 3mol��2����VA2/100 5:4 �ڴ��� ��ABD
��������
�����������1�����ݢ�C(s)+O2(g)�TCO2��g����H1=-393.5kJ•mol-1����2H2��g��+O2��g���T2H2O��g����H2=-483.6kJ•mol-1����C��s��+H2O��g���TCO��g��+H2��g����H3=+131.3kJ•mol-1�����Ȼ�ѧ����ʽ�����ø�˹���ɣ�����-�ۿɵ÷�ӦCO(g)+H2(g)+O2��g���TH2O(g)+CO2(g)����H=-393.5kJ•mol-1-131.3kJ•mol-1=-524.83kJ•mol-1��CO��H2��������Ӧ�ķ���ʽ�ֱ�Ϊ��2CO+O2 2CO2��2H2+O2
2CO2��2H2+O2 2H2O���ӷ���ʽ���Կ�����ͬ���ʵ�����CO��H2ȼ��ת�Ƶĵ�����Ŀ��ȣ����Ա�״����CO��H233.6L��������Ӧ����CO2��H2Oת�Ƶĵ��ӵ����ʵ���Ϊ���x(33.6L)/(22.4L/mol)�y��2=3mol����2���ٸ���ͼ����Կ�����A��ʱCO��ת����Ϊ50%����ƽ��ʱ�����ʵ���Ũ��Ϊ��c(CO)=��5mol/L��/VA��c(H2)=(10mol/L)/VA��c��CH3OH��=(5mol/L��/VA������ƽ�ⳣ��Ϊ=c(CH3OH)/c(CO)��c2(H2)��[(5mol/L��/VA]/[(5mol/L��/VA]��[(10mol/L)/VA]2��VA2/100������ͼ����Կ�����A��ʱCO��ת����Ϊ50%����ƽ��ʱCO��H2��CH3OH�����ʵ����ֱ�Ϊ5mol��10mol��5mol������ͼ����Կ�����A��ʱCO��ת����Ϊ70%����ƽ��ʱCO��H2��CH3OH�����ʵ����ֱ�Ϊ3mol��6mol��7mol��A��B����ʱ���������ʵ����ʵ���֮��Ϊn(A)����n(B)��=20:16=5:4 �������¶ȣ���Ӧ��������Ӧ��ʼ����ƽ��״̬�����ʱ��Ҫ�٣����Է�Ӧ��ʼ����ƽ��״̬�����ʱ��tA ����tC�������ת����Ӧʹƽ��������Ӧ�����ƶ������ݷ�Ӧ����ʽ�������������жϳ��ɲ�ȡ�Ĵ�ʩΪ�����¡���ѹ�����״��ӻ����ϵ�з���������ʴ�Ϊ�����¡���ѹ�����״��ӻ����ϵ�з��������
2H2O���ӷ���ʽ���Կ�����ͬ���ʵ�����CO��H2ȼ��ת�Ƶĵ�����Ŀ��ȣ����Ա�״����CO��H233.6L��������Ӧ����CO2��H2Oת�Ƶĵ��ӵ����ʵ���Ϊ���x(33.6L)/(22.4L/mol)�y��2=3mol����2���ٸ���ͼ����Կ�����A��ʱCO��ת����Ϊ50%����ƽ��ʱ�����ʵ���Ũ��Ϊ��c(CO)=��5mol/L��/VA��c(H2)=(10mol/L)/VA��c��CH3OH��=(5mol/L��/VA������ƽ�ⳣ��Ϊ=c(CH3OH)/c(CO)��c2(H2)��[(5mol/L��/VA]/[(5mol/L��/VA]��[(10mol/L)/VA]2��VA2/100������ͼ����Կ�����A��ʱCO��ת����Ϊ50%����ƽ��ʱCO��H2��CH3OH�����ʵ����ֱ�Ϊ5mol��10mol��5mol������ͼ����Կ�����A��ʱCO��ת����Ϊ70%����ƽ��ʱCO��H2��CH3OH�����ʵ����ֱ�Ϊ3mol��6mol��7mol��A��B����ʱ���������ʵ����ʵ���֮��Ϊn(A)����n(B)��=20:16=5:4 �������¶ȣ���Ӧ��������Ӧ��ʼ����ƽ��״̬�����ʱ��Ҫ�٣����Է�Ӧ��ʼ����ƽ��״̬�����ʱ��tA ����tC�������ת����Ӧʹƽ��������Ӧ�����ƶ������ݷ�Ӧ����ʽ�������������жϳ��ɲ�ȡ�Ĵ�ʩΪ�����¡���ѹ�����״��ӻ����ϵ�з���������ʴ�Ϊ�����¡���ѹ�����״��ӻ����ϵ�з��������
���㣺������ԭ��Ӧ�ĵ���ת����Ŀ���㣻�ø�˹���ɽ����йط�Ӧ�ȵļ��㣻��ѧƽ�⡣



 CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
 CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��