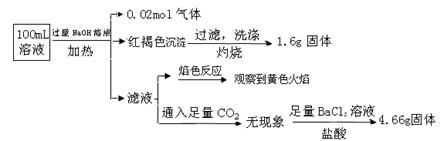
��Ŀ����
��25��ʱ������HA���MOH��������.
��1����0.01mol/L��ǿ��HA��0.01mol/Lǿ��MOH��ϣ���������Һ�� ������ԡ��������ԡ����ԡ�����ͬ���÷�Ӧ�����ӷ���ʽΪ
��2����PH=3��ǿ��HA��PH=11������MOH��ϣ���������Һ�� �������ǣ�
��3����0.01mol/L��ǿ��HA��0.01mol/L����MOH��ϣ���������Һ�� ��������һ��������ӷ���ʽ��
��1����0.01mol/L��ǿ��HA��0.01mol/Lǿ��MOH��ϣ���������Һ�� ������ԡ��������ԡ����ԡ�����ͬ���÷�Ӧ�����ӷ���ʽΪ
��2����PH=3��ǿ��HA��PH=11������MOH��ϣ���������Һ�� �������ǣ�
��3����0.01mol/L��ǿ��HA��0.01mol/L����MOH��ϣ���������Һ�� ��������һ��������ӷ���ʽ��
��1������ H++OH-=H2O
��2������ ��HA���MOH�кͺ��������������OH-(2�֣�����������Ҳ�ɸ���)
��3�� ���� M++H2O MOH+H+(2��)
MOH+H+(2��)
��2������ ��HA���MOH�кͺ��������������OH-(2�֣�����������Ҳ�ɸ���)
��3�� ���� M++H2O
 MOH+H+(2��)
MOH+H+(2��)�����������1����0.01mol/L��ǿ��HA��0.01mol/Lǿ��MOH��ϣ�����ǡ����ȫ��Ӧ����ǿ��ǿ����MA����������Һ�����ԣ��÷�Ӧ�����ӷ���ʽΪH++OH-=H2O����2����PH=3��ǿ��HA��PH=11������MOH��ϣ���������Һ�Լ��ԣ������ǣ�PH=3��ǿ��HA��PH=11������MOH�кͺ��������������OH-����3����0.01mol/L��ǿ��HA��0.01mol/L����MOH��ϣ�����ǡ����ȫ��Ӧ����ǿ��������MA������������ˮ�������ԣ�������һ��������ӷ���ʽ��
M++H2O
 MOH+H+��
MOH+H+��
��ϰ��ϵ�д�
 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ
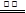 2Cu�� +O2�� +4H+
2Cu�� +O2�� +4H+ Fe(OH)3(����)+3H+
Fe(OH)3(����)+3H+